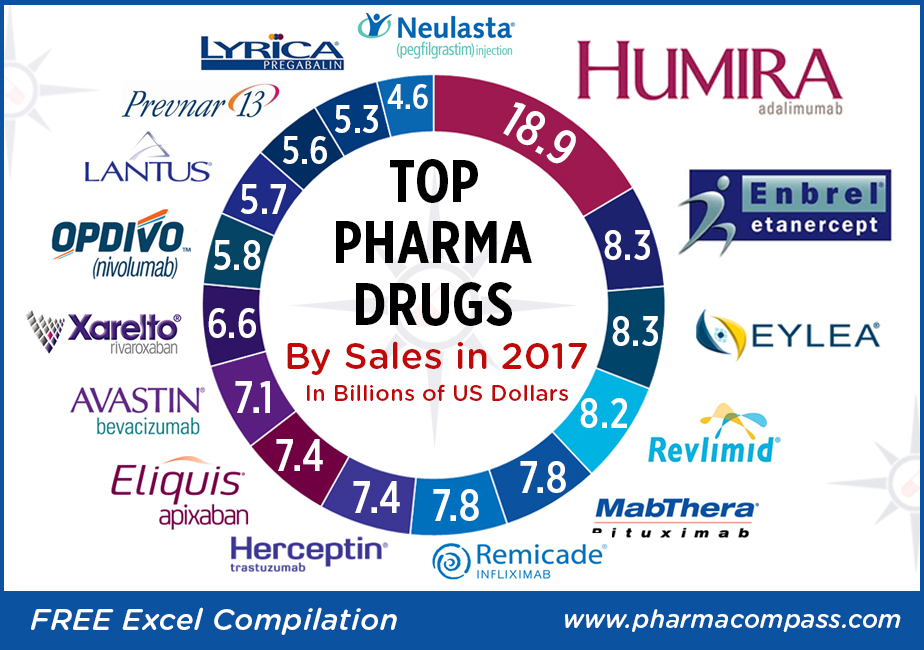
The year 2017 was a landmark year for pharmaceutical industries in the US and Europe, with a sharp increase in the number of new molecular entities (NMEs) being approved in both geographies.
The US Food and Drug Administration (USFDA) approved 46 NMEs in 2017, the second highest since 1996 when 53 NMEs were approved. In Europe, the European Medicines Agency (EMA) approved 35 drugs with a new active substance, up from 27 in 2016.
Sales for most major pharmaceutical companies continued to grow in 2017. Earnings forecasts for 2018 have been raised due to the recent US tax reform that has generated investor hopes for accelerated dividend growth and share buyback plans.
Top-sellers: Humira races ahead, despite launch of biosimilars; Enbrel a distant second
There wasn’t any upheaval at the top of the pharma drug sales charts. AbbVie’s anti-TNF (tumor necrosis factor) giant Humira (adalimumab), which is approved to treat psoriasis and rheumatoid arthritis, added almost another US $3 billion to its 2016 sales and posted nearly US $19 billion in revenues.
Last year, AbbVie’s raised expectations for Humira’s earnings to reach US $21 billion in global sales by 2020. The company believes this drug will continue to be a significant cash contributor until 2025 and the US $21 billion sales forecast by 2020 is about US $3 billion higher than its expectation two years ago.
In 2016, the US Food and Drug Administration (FDA) approved Amgen’s Amjevita (adalimumab-atto) — a biosimilar of Humira. And in 2017, another Humira biosimilar — Boehringer Ingelheim’s Cyltezo (adalimumab-adbm) — received approval from the FDA and European authorities.