- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
Pharmaceutical Excipients - Some Definition
There are several explanations to pharmaceutical excipients covering origin, regulatory and functionality aspects.
“The word excipient is derived from the Latin excipere, meaning 'to except', which is simply explained as 'other than'. Pharmaceutical excipients are basically everything other than the active pharmaceutical ingredient. Ideally, excipients should be inert, however, recent reports of adverse reactions have suggested otherwise.” (Australian Prescriber)
"Pharmaceutical excipients are substances other than the active pharmaceutical ingredient (API) that have been appropriately evaluated for safety and are intentionally included in a drug delivery system.”
Simply said the excipients enable the drug substance to be applied to the patient in the right form and supports the way and place of action without being active themselves.
Ideal Excipient Properties
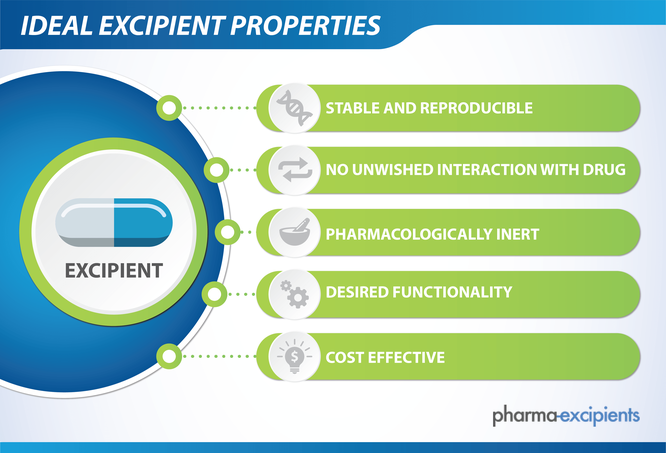
Excipients range from inert and simple to active and complex substances that can be difficult to characterize. Traditionally, excipients were often structurally simple, biologically inert, and of natural origin, such as corn, wheat, sugar, and minerals. Many more novel and increasingly complex excipients have been developed as novel drug formulation delivery systems emerge and evolve. The inert and innocuous nature of excipients is no longer a given feature in drug formulations. Many excipients are potential toxicants at high doses in animals, though safe in humans at therapeutic doses, including commonly used excipients such as cyclodextrins, dextran, and polyethylene glycol.
Roles of Excipients

Excipients have different roles in a formulation. Some of the major ones can be:
- Aid in the processing of the drug delivery system during its manufacture
- Protect, support, or enhance stability, bioavailability, or patient acceptability,
- Assist in product identification, and enhance any attribute of the overall safety
- Assist in the effectiveness and/or delivery of the drug in use
- Assist in maintaining the integrity of the drug product during storage
Categorization of Excipients
Pharmaceutical excipients can be categorized in different ways. Such as
By route of Administration
- Oral Excipients
- Topical Excipients
- Parenteral Excipients
- Other Excipients
By origin
Inorganic Chemicals
- Calcium Phosphates
- Calcium Carbonate
- Calcium Sulfate
- Halites
- Metallic Oxides
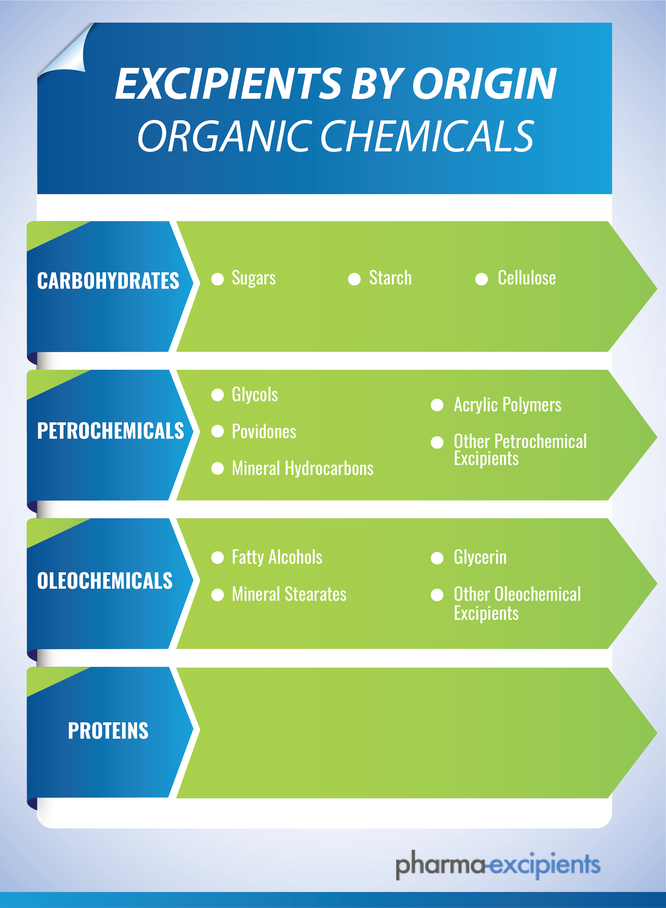
Organic Chemicals
- Carbohydrates
- Sugars
- Actual Sugars
- Sugar Alcohols
- Artificial Sweeteners
- Starch
- Modified Starch
- Dried Starch
- Converted Starch
- Cellulose
- Cellulose Ethers
- Cellulose Esters
- CMC and Croscarmellose Sodium
- Microcrystalline Cellulose
- Petrochemicals
- Glycols
- Polyethylene Glycol
- Propylene Glycol
- Povidones
- Mineral Hydrocarbons
- Petrolatum
- Mineral Waxes
- Mineral Oils
- Acrylic Polymers
- Other Petrochemical Excipients
- Oleochemicals
-
- Fatty Alcohols
- Mineral Stearates
- Glycerin
- Other Oleochemical Excipients
- Proteins
Excipients by Origin: Organic Chemicals
By Functionality
- Fillers & Diluents
- Binders
- Suspension & Viscosity Agents
- Coatings
- Flavoring Agents
- Disintegrants
- Colorants
- Lubricants & Glidants
- Preservatives
- Etc.
Main Excipients
Oral solid dosage forms are still the majority of dosage forms in the pharmaceutical industry mainly driven by convenience of administration, stability, formulation, transport and other advantages over other forms such as injectables, topical, liquid or suppositories.
The main excipients are therefore also to be found in the field of solid dosage formulation and nowadays often looked at as commodities by some parts of the industry.
- Magnesium Stearate
- Microcrystalline Cellulose
- Starch (corn)
- Silicone/Titanum Dioxide
- Stearic Acid
- Sodium Starch Glycolate
- Gelatin
- Talc
- Sucrose
- Calcium Stearate
- Povidone
- Pregelantinized Starch
- HPMC
- OPA products
- Croscarmellose
- Hydroxypropyl Cellulose
- Ethycellulose
- Calcium Phosphate
- Crospovidone
- Shellac (and Glaze)
Theses excipients are the most used in quantity but are under pressure by price and competition as different manufacturers are on the market with the same product / product types - at least based on the specifications given by the official monographs. Same might not be the same as the applied manufacturing process or different raw materials can have an effect on the excipient characteristics which is not reflected in the monograph testing. A well-known example is the different water uptake capacity of Microcrystalline Cellulose based on the drying process applied during production which can have an influence in wet granulation or extrusion/spheronzation.
