- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
What are Sweeteners?
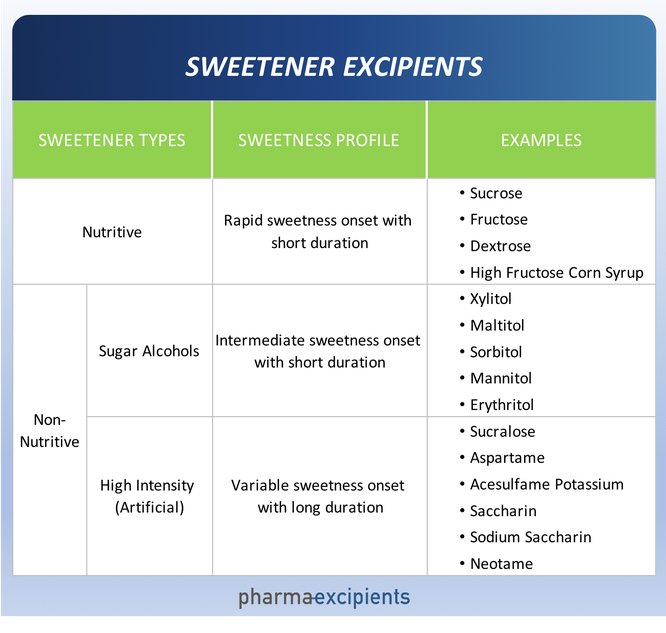
There are a multitude of sweeteners available to the pharmaceutical scientist. Sweeteners may be broadly grouped into two categories – nutritive and non-nutritive. Nutritive sweeteners deliver calories and as their name suggests, non-nutritive do not. Non-nutritive sweeteners can be further characterized as bulk (sugar alcohols) and high intensity (artificial).
Some excipients that are sweet are often incorrectly listed in publications as “sweeteners.” These are excipients used primarily for purposes other than imparting sweetness, for example, co-solvents such as glycerol or propylene glycol, and bulking agents such as lactose or maltodextrin. The most common examples of excipients used for the purpose of sweetening drug formulations are shown below.
How to Select a Sweetener
The selection of candidate sweeteners is informed by technical, regulatory and clinical considerations.
Technical Considerations
There are numerous technical issues that need to be considered in identifying candidate sweeteners for the formulation design space. These include:
-
Sweetness Profile. The sweetness profile (intensity, onset and duration) of each sweetener is different as summarized in the table above. The
intensity and duration of the aversive attribute of the drug active (bitter, salty, or sour) informs identification of candidate sweeteners. For example, many drug actives have a long-lived
bitterness profile that for palatability requires the lingering sweetness afforded by many artificial sweeteners.
-
Physical Properties. Nutritive sweeteners and sugar alcohols have low relative sweetness and are sometimes employed as fillers in dosage forms
requiring additional mass, such as tablets and powder sachets. Due to this property, sugar alcohols and nutritive sweeteners are collectively known as “bulk sweeteners”. Many drug
formulations require combinations of bulk and high intensity sweeteners to provide the requisite sweetness profile. Some bulk sweeteners have added benefits (e.g. improved freeze/thaw
stability) and disadvantages (e.g. tendency to crystalize on bottle cap threads of multi-dose containers) that should also be taken into consideration.
-
Microbiological Stability. Depending on usage level, bulk sweeteners can improve microbiological stability by lowering water activity, reducing
the need for chemical preservatives.
- Chemical Stability. The artificial sweeteners typically exhibit good solid-state stability across the board, but some have poor stability in solution. For example, aspartame readily hydrolyzes in aqueous environments, and a liquid aspartame-sweetened product would experience drastic changes by 6 months storage. Some manufacturers do not recommend that sucralose be used in formulations with a pH above 7.
Regulatory Considerations
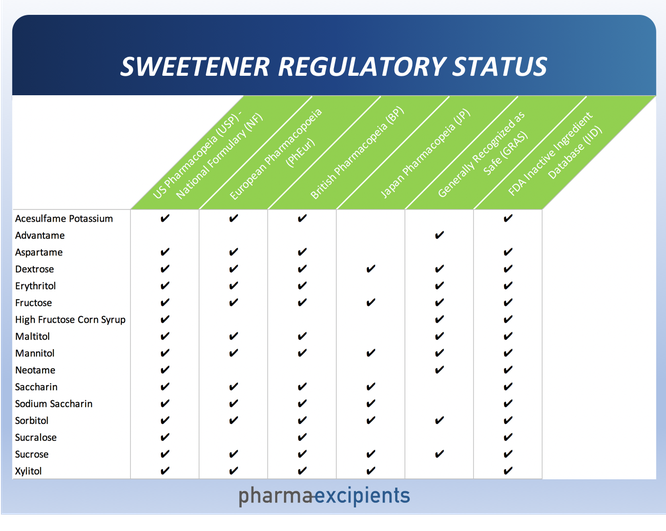
The regulatory acceptability of sweeteners can vary by geography. Many of the common sweeteners are listed in the various pharmacopeia – United States Pharmacopeia and National Formulary (USP-NF), British Pharmacopeia (BP), Japan Pharmacopeia (JP), European Pharmacopoeia (PhEur). Some have been determined to be Generally Recognized as Safe (GRAS) by the manufacturer, or by the Flavor and Extract Manufacturers Association (FEMA) in accordance with the 1958 Food Additives Amendment to the Federal Food, Drug and Cosmetic Act. Additionally, the FDA maintains the Inactive Ingredient Database (IID) which lists excipients with prior precedent of use in approved pharmaceutical formulations.
A high-level summary of the regulatory status of the common sweeteners is shown below.
People who have phenylketonuria (PKU), a rare genetic disorder, have a difficult time metabolizing phenylalanine, a component of both aspartame and advantame - a new artificial sweetener that is chemically related to aspartame.
Interstingly, products containing only aspartame must bear an informational statement for people with PKU alerting them about the presence of phenylalanine. Because advantame is much sweeter than aspartame, only a very small amount needs to be used to reach the same level of sweetness. As a result, products containing advantame do not need to bear that statement. Advantame is FEMA-GRAS but is not in the IID as it has not been used yet in an FDA-approved drug product, nor has it made its way into the major pharmacopeia.
Clinical Considerations
Clinical considerations may weigh against the use of nutritive sweeteners due to co-morbidity issues (e.g., diabetes) and their cariogenic potential, though the latter is often overstated, particularly for life saving/extending drugs. Sugar alcohols can increase gastrointestinal motility but at typical usage levels, this is generally only a concern in very young patients or in special populations such as those undergoing Total Parenteral Nutrition (TPN).
The Importance of Sweeteners
For most drug actives, the sweetener system plays a crucial but generally insufficient role in developing palatable formulations. Other excipients classes such as buffers, salts, taste modifiers and flavors are generally required for effective taste-masking.
Many excipients are sweet; but only a subset has the primary function of imparting sweetness to the formulation, i.e., are true sweeteners. The selection of sweeteners is informed by a myriad of technical, regulatory and clinical considerations. From a taste masking perspective, the sweetness profile – intensity, onset and duration – is critically important.
Blog Post for pharmaexcipients.com - Prepared by Senopsys LLC by David Tisi -- All Rights Reserved
David Tisi is the Technical Director of Senopsys LLC, a specialty services firm dedicated to the development of palatable drug products. He has 15 years of experience in taste assessment and taste masking of investigational and approved drugs for children and adults, leveraging his background in sensory science and food chemistry. He can be reached at david.tisi@senopsys.com