- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. May 2018
The aim of this study was to develop a palatable donepezil (DP) orodispersible film (ODF) to facilitate the swallowing process and investigate the effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Complexation of DP with hydroxypropyl-β-cyclodextrin (HP-β-CD) was applied to mask the bitter taste then the prepared complexes were incorporated into ODF using solvent casting method. The taste-masking efficiency was evaluated by e-tongue; meanwhile the...
17. May 2018
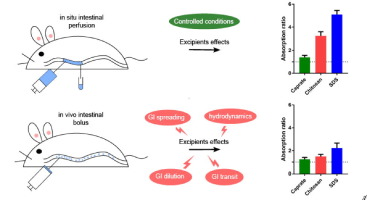
Pharmaceutical excipients that may affect gastrointestinal (GI) drug absorption are called critical pharmaceutical excipients (CPEs), or absorption-modifying excipients (AMEs) if they act by altering the integrity of the intestinal epithelial cell membrane. Some of these excipients increase intestinal permeability, and subsequently the absorption and bioavailability of the drug. This could have implications for both the assessment of bioequivalence and the efficacy of the absorption-enhancing...
24. April 2018
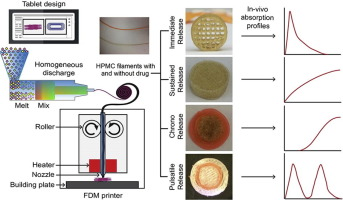
Three-dimensional printing (3DP), though developed for nonmedical applications and once regarded as futuristic only, has recently been deployed for the fabrication of pharmaceutical products. However, the existing feeding materials (inks and filaments) that are used for printing drug products have various shortcomings, including the lack of biocompatibility, inadequate extrudability and printability, poor drug loading, and instability. Here, we have sought to develop a filament using a single...
02. March 2018
For orally administered drugs, the rate and extent of absorption are governed by the physiology of the gastrointestinal tract, the characteristics of the dosage form and the physico-chemical properties of the drug. This thesis primarily aimed to improve the mechanistic understanding and the predictability of processes involved in the absorption of orally administered drugs using a population modeling approach. A secondary aim was to propose an optimized dosing regimen for first line anti-tubercu
01. October 2017
Vitamin E TPGS (TPGS) has both surfactant and P-glycoprotein (P-gp) inhibitory effects. While surfactants were previously found to cause solubility-permeability tradeoff, TPGS P-gp inhibitory effects may change this unfavorable interplay. The purpose of this research was to investigate the solubility-permeability interplay when using TPGS vs. amorphous solid dispersions (ASD) as oral drug delivery systems for the anticancer, P-gp substrate, lipophilic drug etoposide. The concentration-dependent...
06. September 2017
Abstract: Liquid crystal (LC)-forming lipids represent an important class of biocompatible amphiphiles and their application extends to cosmeceutical, dietary, and pharmaceutical technologies. In the present study, we aimed to develop strategies for designing and optimizing oral and topical LC formulations by evaluating their in vitro and in vivo drug absorption performances. C17-Monoglycerol ester (MGE) was used as a LC-forming lipid. p-Amino benzoic acid, methyl PABA, ethyl PABA, and sodium...
04. September 2017
This work presents a review of literature and experimental data relevant to the possibility of waiving pharmacokinetic bioequivalence studies in human volunteers for approval of immediate release solid oral pharmaceutical forms containing folic acid as the single active pharmaceutical ingredient. For dosage forms containing 5 mg folic acid, the highest dose strength on the WHO Essential Medicines List, the dose/solubility ratio calculated from solubility studies was higher than 250 mL,...
28. August 2017
The objective of the present study was to enhance the solubility, dissolution and hence anti-inflammatory activity of poorly soluble drug indomethacin (IMN) by formulating into self emulsifying systems.
14. August 2017
Insulin, a hormonal drug for the management of diabetes mellitus has continued to attract attention of many researchers and pharmaceutical formulators for more than a decade. However, its low oral bioavailability due to the activities of intestinal enzymes restricts its route of administration to only the parenteral route.
03. March 2017
Abstract The study aimed to characterise the mechanism of release and absorption of Basmisanil, a biopharmaceutics classification system (BCS) class 2 compound, from immediate-release formulations via mechanistic absorption modelling, dissolution testing, and Raman imaging. An oral absorption model was developed in GastroPlus® and verified with single-dose pharmacokinetic data in humans. The properties and drug release behaviour of different oral Basmisanil formulations were characterised via...