- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
02. October 2018
The formulation of amorphous solid dispersions (ASDs) is an effective way to improve the bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs). The combination of an amorphous state of the drug and the presence of crystallization-inhibiting polymers retains a high amount of dissolved API over time. ASDs with ketoconazole and different polymers were manufactured by spray drying and their characteristics as well as performance were analyzed. Dissolution tests with a...
26. September 2018
The increasing number of poorly water-soluble drug candidates in pharmaceutical development is a major challenge. Enabling techniques such as amorphization of the crystalline drug can result in supersaturation with respect to the thermodynamically most stable form of the drug, thereby possibly increasing its bioavailability after oral administration. The ease with which such crystalline drugs can be amorphized is known as their glass forming ability (GFA) and is commonly described by the...
05. September 2018
The predictability of preformulation screening tools for polymer selection in amorphous solid dispersions (ASD) regarding supersaturation and precipitation was systematically examined. The API-polymer combinations were scaled up by means of hot-melt extrusion and spray-drying to verify the predictions. As there were discrepancies between a solvent-based screening and performance of ASD, a new screening tool with improved predictability at minimal investments of time and material is presented....
30. August 2018
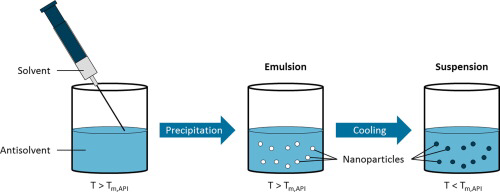
Antisolvent precipitation of poorly water-soluble drugs is a promising formulation technique to synthesize amorphous nanoparticles. The dissolution behavior of these nanoparticles is improved because of the high specific surface area and the amorphous state, leading to an enhanced bioavailability of the drug molecules. Nevertheless, stabilization of precipitated drug nanoparticles against agglomeration and recrystallization, which constitutes a key issue for further processing steps, has turned...
25. August 2018
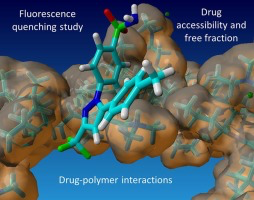
Solid dispersions (SDs) represent an important formulation technique to achieve supersaturation in gastro-intestinal fluids and to enhance absorption of poorly water-soluble drugs. Extensive research was leading to a rather good understanding of SDs in the dry state, whereas the complex interactions in aqueous medium are still challenging to analyze. This paper introduces a fluorescence quenching approach together with size-exclusion chromatography to study drug and polymer interactions that...
18. August 2018
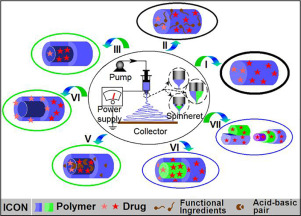
The development of oral dosage forms for poorly water-soluble active pharmaceutical ingredients (APIs) is a persistent challenge. A range of methods has been explored to address this issue, and amorphous solid dispersions (ASDs) have received increasing attention. ASDs are typically prepared by starting with a liquid precursor (a solution or melt) and applying energy for solidification. Many techniques can be used, with the emergence of electrospinning as a potent option in recent years. This...
16. August 2018
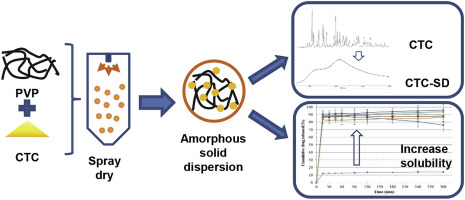
Chlortetracycline hydrochloride (CTC) has been reported as having low aqueous solubility, leading to a limitation in its administration in treatments. This study demonstrated a strategy to enhance the supersaturated solubility of CTC by amorphous solid dispersion (SD). CTC-SDs were prepared with hydrophilic polymers, polyvinylpyrrolidone (PVP) and copovidone using various preparation methods: water bath evaporation, speed vacuum evaporation, and spray drying. Physicochemical properties and...
27. July 2018
The present study elucidated the advantages of using hydroxypropyl methylcellulose E5 (HPMC) as auxiliary excipient in maintaining storage stability and solubilization ability for curcumin amorphous solid dispersion (Cur ASDs) formulated by Eudragit E100 (E100). Polarized light microscopy and in vitro dissolution experiment was applied for confirming the ability of HPMC on inhibiting crystallization thereby maintaining storage stability in Cur ASDs. Meanwhile, as a non-ionic surfactant, HPMC...
07. June 2018
Poly(2-ethyl-2-oxazoline) (PEOX), a biocompatible polymer considered as pseudo polypeptide, was introduced as a potential alternative to the commonly used polymer, poly(vinylpyrrolidone) (PVP) for the preparation of solid dispersion with a poorly soluble drug. Glipizide (GPZ), a BCS class II model drug, was selected for solubility and dissolution rate study. GPZ-polymer solid dispersions and physical mixtures were characterized and investigated by X-Ray diffractometry, differential scanning...
14. May 2018
The objectives of this study were to explore sodium dodecyl sulfate (SDS) and Soluplus on the crystallization inhibition and dissolution of felodipine (FLDP) extrudates by bottom-up and top-down approaches. FLDP extrudates with Soluplus and/or SDS were prepared by hot melt extrusion (HME), and characterized by PLM, DSC and FT-IR. Results indicated that Soluplus inhibited FLDP crystallization and the whole amorphous solid dispersions (ASDs) were binary FLDP-Soluplus (1:3) and ternary...