- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
24. August 2018
In today's drug development world, combinatorial chemistry, high-throughputscreening, and genomics have provided a technologic platform that produces a large number of new chemical entities withtherapeutic potential each year. Its outcome the new chemical entities shifted towards higher molecular weight and increasing lipophilicity that results in poor water solubility which primarily affectsthe bioavailability of orally administered drugs. Hence, the poor aqueoussolubility not only limits the...
31. July 2018
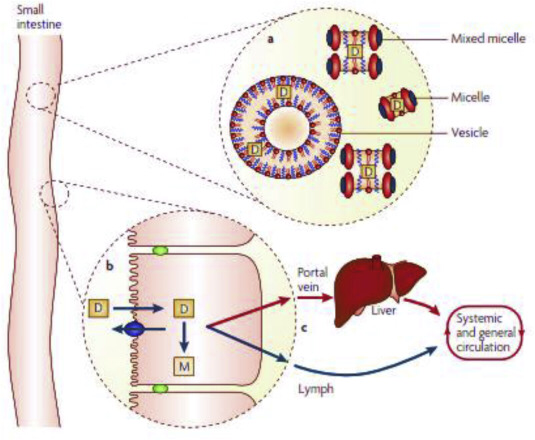
Poor aqueous solubility is a major challenge in today's biopharmaceutics. While solubility-enabling formulations can significantly increase the apparent solubility of the drug, the concomitant effect on the drug's apparent permeability has been largely overlooked. The mathematical equation to describe the membrane permeability of a drug comprises the membrane/aqueous partition coefficient, which in turn is dependent on the drug's apparent solubility in the GI milieu, suggesting that the...
30. July 2018
Drug nanoparticles embedded in a dispersant matrix as a secondary phase, i.e., drug-laden nanocomposites, offer a versatile delivery platform for enhancing the dissolution rate and bioavailability of poorly water-soluble drugs. Drug nanoparticles are prepared by top-down, bottom-up, or combinative approaches in the form of nanosuspensions, which are subsequently dried to prepare drug-laden nanocomposites. In this comprehensive review paper, the term “nanocomposites” is used in a broad...
04. September 2017
This work presents a review of literature and experimental data relevant to the possibility of waiving pharmacokinetic bioequivalence studies in human volunteers for approval of immediate release solid oral pharmaceutical forms containing folic acid as the single active pharmaceutical ingredient. For dosage forms containing 5 mg folic acid, the highest dose strength on the WHO Essential Medicines List, the dose/solubility ratio calculated from solubility studies was higher than 250 mL,...
02. March 2017
Abstract The aim of this work was to develop self-nanoemulsifying liquisolid tablets (SNELT) to enhance the dissolution profile of poorly water-soluble simvastatin. SNELT present a unique technique of incorporating self-nanoemulsifying drug delivery systems (SNEDDS) into tablets. Optimized SNEDDS containing different oils, Cremophor® RH 40 (surfactant) and Transcutol® HP (co-surfactant), at different ratios, were used as liquid vehicles and loaded on carrier material, microcrystalline...
13. February 2017
Abstract The objective of this study is to enhance the dissolution properties of leflunomide, a class BCS-II drug by incorporating the self emulsifying (SE) form of the drug onto liquisolid systems in the form of tablets. Different formulae were prepared by dissolving leflunomide in PEG300 then forming SE systems using tween 80 as surfactant and either sesame oil and paraffin oil then adsorbing on powder excipients to form SE liquisolid powders. The prepared powders showed adequate flowability....
07. June 2016
Purpose Currently, the FDA allows biowaivers for Class I (high solubility and high permeability) and Class III (high solubility and low permeability) compounds of the Biopharmaceutics Classification System (BCS). Scientific evidence should be provided to support biowaivers for BCS Class I and Class III (high solubility and low permeability) compounds.
31. March 2016
The objective was to assess the impact of larger than conventional amounts of 14 commonly used excipients on Biopharmaceutics Classification System (BCS) class 3 drug absorption in humans. Cimetidine and acyclovir were used as model class 3 drugs across three separate four-way crossover bioequivalence (BE) studies (n ¼ 24 each) in healthy human volunteers, denoted as study 1A, 1B, and 2. In study 1A and 1B, three capsule formulations of each drug were manufactured, collectively involving 14...
23. May 2015
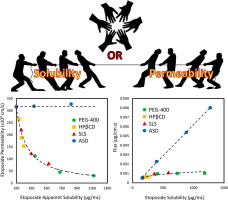
The purpose of this study was to conduct a head-to-head comparison of different solubility-enabling formulations, and their consequent solubility–permeability interplay. The low-solubility anticancer drug etoposide was formulated in several strengths of four solubility-enabling formulations: hydroxypropyl-β-cyclodextrin, the cosolvent polyethylene glycol 400 (PEG-400), the surfactant sodium lauryl sulfate, and an amorphous solid dispersion formulation......