- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. July 2018
The number of biologics in the therapeutic development pipeline is increasing including those delivered though inhalation (Morales, 2017; Fathe, 2016). Biologics comprise a broad variety of complex macromolecules with unique physicochemical characteristics. These distinctive characteristics control their pharmacological mechanisms of action, stability, and ultimately affect their processing, formulation, and delivery requirements. This review systematically covers crucial aspects of biologic...
30. July 2018
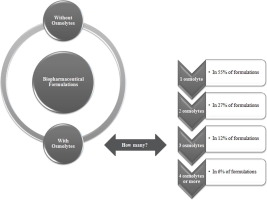
Osmolytes are small organic molecules accumulated by cells in response to environmental stresses. They are represented by amino acids, sugars, polyols, tertiary sulphonium and quaternary ammonium compounds. These molecules present a protective behaviour and favour the equilibrium of macromolecules towards the native form, preventing denaturation and promoting the folding of unfolded proteins. Protein formulations due to their biological character require greater care during the manufacturing...
13. February 2018
Hydrogels are, from a commercial perspective especially because of their ease of production, attractive sustained-release systems for high potent immunoglobulins with short circulation half-lives. Hydrogel formulations can reduce the dosing frequency while maintaining therapeutically relevant drug concentrations locally as well as systemically.
09. February 2018
Modern medicine, biological research, and clinical diagnostics depend on the reli- able supply and storage of complex biomolecules. However, biomolecules are inherently susceptible to thermal stress and the global distribution of value-added biologics, including vaccines, biotherapeutics, and Research Use Only (RUO) proteins, requires an integrated cold chain from point of manufacture to point of use. To mitigate reliance on the cold chain, formulations have been engineered to protect biologics
15. January 2018
Current limitations to on-demand drug manufacturing can be addressed by technologies that streamline manufacturing processes. Combining the production of two or more drugs into a single batch could not only be useful for research, clinical studies, and urgent therapies but also effective when combination therapies are needed or where resources are scarce.
27. June 2017
For decades, everybody has become familiar with many brand-name and generic chemically synthesized drugs that are small molecule drug products, such as amoxicillin, Lipitor, and Crestor. Biological products, on the other hand, are practically and conceptually different as a class of therapeutic medicinal products.
22. July 2016
Mir Imran, from Rani Therapeutics™, provides an exclusive update on his company's groundbreaking robotic Auto-Pill™, which delivers a pain-free intestinal injection using a dissolvable needle made from materials that can be absorbed or easily passed out of the body. This approach allows the delivery of biologics of any molecular weight. Link to ONdrugDelivery Magazine
22. April 2016
The main route of administration for drug products is the oral route, yet biologics are initially developed as injectables due to their limited stability through the gastrointestinal tract and solubility issues. In order to avoid injections, a myriad of investigations on alternative administration routes that can bypass enzymatic degradation and the first-pass effect are found in the literature. As an alternative site for biologics absorption, the buccal route presents with a number of...
29. February 2016
Despite decades of effort, oral delivery of peptide, protein and antibody drugs remains a major pharmaceutical challenge, with only a handful of products on the market. That is particularly disappointing as biologics are also the fastest growing segment of the pharma market, tripling in value from $36bn to $163bn between 2001 and 2012, thanks to their high specificity for drug targets. Their specificity derives from their structural complexity, which is also the reason they are so challenging...
24. May 2015
Formulation Strategies for Different Stages of Development, New Product Formats, Device/Delivery Systems and Emerging Analytical Methods January 18-19, 2016 http://www.chi-peptalk.com/biologics-formulation/