- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. July 2018
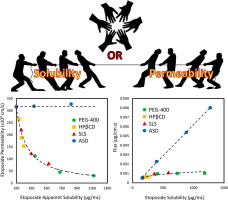
Poor aqueous solubility is a major challenge in today's biopharmaceutics. While solubility-enabling formulations can significantly increase the apparent solubility of the drug, the concomitant effect on the drug's apparent permeability has been largely overlooked. The mathematical equation to describe the membrane permeability of a drug comprises the membrane/aqueous partition coefficient, which in turn is dependent on the drug's apparent solubility in the GI milieu, suggesting that the...
22. February 2018
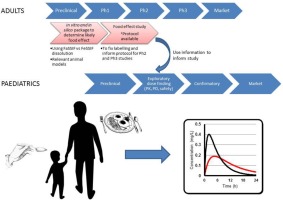
A small amount of food is commonly used to aid administration of medicines to children to improve palatability and/or swallowability. However the impact of this co-administered food on the absorption and subsequent pharmacokinetic profile of the drug is unknown. Existing information on food effects is limited to standard protocols used to evaluate the impact of a high fat meal in an adult population using the adult medication. In the absence of a substantial body of data, there are no specific...
04. September 2017
This work presents a review of literature and experimental data relevant to the possibility of waiving pharmacokinetic bioequivalence studies in human volunteers for approval of immediate release solid oral pharmaceutical forms containing folic acid as the single active pharmaceutical ingredient. For dosage forms containing 5 mg folic acid, the highest dose strength on the WHO Essential Medicines List, the dose/solubility ratio calculated from solubility studies was higher than 250 mL,...
11. July 2017
This article discusses the range of outcomes from biopharmaceutical studies of specific modified release (MR) product examples in preclinical models and humans.
04. January 2017
Active ingredients in pharmaceuticals di er by their physico-chemical properties and their bioavailabil- ity therefore varies. e most frequently used and most convenient way of administration of medicines is oral, however many drugs are little soluble in water. us they are not su ciently e ective and suitable for such administration. For this reason a system of lipid based formulations (LBF) was developed. Series of formula- tions were prepared and tested in water and biorelevant media. On the...
18. July 2016
OBJECTIVES: The aim of this study was to determine the influence of non-ionisable excipients hydroxypropyl-β-cyclodextrin (HPβCD) and poloxamers 407 and 188 on the supersaturation and precipitation kinetics of ibuprofen, gliclazide, propranolol and atenolol induced through solution pH shifts using the CheqSol method. METHODS: The drug's kinetic and intrinsic aqueous solubilities were measured in the presence of increasing excipient concentrations using the CheqSol method. Experimental data...
31. March 2016
Abstract We previously concluded that 12 common excipients need not be qualitatively the same and quantitatively very similar to reference for Biopharmaceutics Classification System–based biowaivers. This conclusion for regulatory relief is based upon a series of bioequivalence studies in humans involving cimetidine and acyclovir. Limitations were also discussed. We understand the major concern of García-Arieta et al. is that “results obtained by Vaithianathan et al. should not be...
31. March 2016
The objective was to assess the impact of larger than conventional amounts of 14 commonly used excipients on Biopharmaceutics Classification System (BCS) class 3 drug absorption in humans. Cimetidine and acyclovir were used as model class 3 drugs across three separate four-way crossover bioequivalence (BE) studies (n ¼ 24 each) in healthy human volunteers, denoted as study 1A, 1B, and 2. In study 1A and 1B, three capsule formulations of each drug were manufactured, collectively involving 14...
05. February 2016
PURPOSE: Compound solubility serves as a surrogate indicator of oral biopharmaceutical performance. Between infancy and adulthood, marked compositional changes in gastrointestinal (GI) fluids occur. This study serves to assess how developmental changes in GI fluid composition affects compound solubility. METHODS: Solubility assessments were conducted in vitro using biorelevant media reflective of age-specific pediatric cohorts (i.e., neonates and infants). Previously published adult media...
01. February 2016
Poor water solubility of many drugs has emerged as one of the major challenges in the pharmaceutical world. Polymer-based amorphous solid dispersions have been considered as the major advancement in overcoming limited aqueous solubility and oral absorption issues. The principle drawback of this approach is that they can lack necessary stability and revert to the crystalline form on storage. Significant upfront development is, therefore, required to generate stable amorphous formulations. A...