- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
12. September 2018
The amorphous solid dispersion (ASD) technique has been employed to formulate poorly-soluble drugs, however, development of solid dosage forms with ASD is challenging due to the high propensity of amorphous drug to precipitate upon dissolution. Thus this work aimed to explore the potential of controlled release amorphous solid dispersion (CRASD) systems using polyvinyl acetate (PVAc) as a release-retarding excipient to mitigate the drug precipitation during dissolution of poorly water-soluble...
10. May 2018
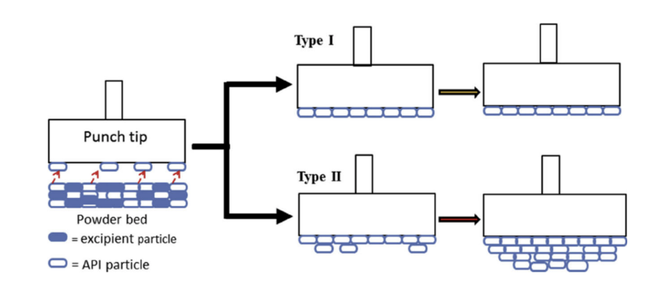
Purpose To investigate how excipient matrix affects punch sticking propensity of active pharmaceutical ingredients (API), with the focus on the effect of bonding interactions between API-API (F2) and API-excipient (F3). Method Sticking kinetics of direct compression formulations, consisting of 20% of celecoxib (CEL) or ibuprofen (IBN) indifferent excipient matrices, i. e., microcrystalline cellulose (Avice l PH102 and Avicel PH105 dry coated with nano-sized silica (PH105(n)), hypromellose (K15...
01. December 2017
The low aqueous solubility of celecoxib (CCB) hampers its oral bioavailability and permeation from aqueous environment through biological membranes. The aim of this study was to enhance the aqueous solubility of CCB by complexation with cyclodextrin (CD) in the presence of water-soluble polymer. The effects of different CDs (αCD, βCD, γCD, 2-hydroxypropyl-β-cyclodextrin and randomly methylated β-cyclodextrin (RMβCD)) and mucoadhesive, water-soluble polymers (hydroxypropyl methylcellulose (HPMC),
05. October 2017
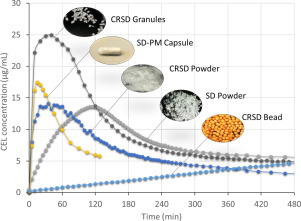
The purpose of this study was to develop and evaluate a dry elixir (DE) system for enhancing the dissolution rate and oral bioavailability of celecoxib. DE system has been used for improving solubility, oral bioavailability of poorly water-soluble drugs.
24. May 2016
This study investigated the non-sink in vitro dissolution behavior and in vivo performance in rats of celecoxib (CCX) amorphous solid dispersions with polyvinyl acetate (PVA), polyvinylpyrrolidone (PVP) and hydroxypropyl methylcellulose (HPMC) at different drug doses. Both in vitro and in vivo, the amorphous solid dispersions with the hydrophilic polymers PVP and HPMC led to higher areas under both, the in vitro dissolution and the plasma concentration-time curves (AUC) compared to crystalline...
26. December 2015
Biopolymers are naturally occurring polymeric biomolecules. They are easily accessible, relatively cheap, can be synthetically altered and biodegradable. Locust bean gum and Xanthan gum is widely used ingredient in drug formulations due to their non interacting behavior with the drug and less toxicity. Microparticle formulations of celecoxib, a COX-2 inhibitor were formulated using Locust bean gum and Xanthan gum. More
06. September 2015
Objective of this work was to understand the mechanism of formation of celecoxib nanocrystals in celecoxib: mannitol nanocrystalline solid dispersion (NSD). Solution of celecoxib and mannitol was spray dried in 1:1 (g:g) proportion to obtain NSD, with average crystallite size of 214.07 ± 45.27 nm. Solubility parameters of celecoxib and mannitol were 23.1 MPa1/2 and 38.5 MPa1/2, respectively, hinting their immiscibility. More