- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. September 2018
The increasing number of poorly water-soluble drug candidates in pharmaceutical development is a major challenge. Enabling techniques such as amorphization of the crystalline drug can result in supersaturation with respect to the thermodynamically most stable form of the drug, thereby possibly increasing its bioavailability after oral administration. The ease with which such crystalline drugs can be amorphized is known as their glass forming ability (GFA) and is commonly described by the...
26. March 2018
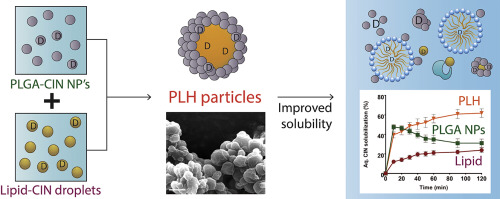
A novel hybrid microparticulate system composed of poly(lactic-co-glycolic) acid (PLGA) nanoparticles and submicron medium-chain triglyceride (MCT) droplets was fabricated to overcome the pH-dependent solubility and precipitation challenges associated with a model poorly water-soluble weak base, cinnarizine (CIN). Molecular CIN was confined within both the lipid and polymer phase of PLGA-lipid hybrid (PLH) and PLGA-lipid-mannitol hybrid (PLMH) particles, which offered significant...
24. February 2016
Cinnarizine (CIN), a poorly soluble drug with erratic bioavailability due to pH dependent solubility has limited advantage to formulate oral solid dosage forms in subject having low gastric acidity. In present study precipitation-ultrasonication was used to fabricate nanosuspensions of cinnarizine stabilized by Poly vinyl alcohol (PVA) to enhance the bioavailability. We investigated the effects of PVA concentration (X1) and solvent to antisolvent ratio (X2) on the quality attributes like mean...
23. January 2016
Cyclodextrins (CDs) are frequently used as an excipient to enhance the intestinal drug absorption of compounds with a low aqueous solubility. However, there exists an intricate interplay between opposing effects that determines the optimal dosing criterion. These opposing effects are the benefits of circumventing the dissolution time required to dissolve the non-absorbable drug particles in the intestine versus the disadvantage of decreasing the concentration of the drug available to permeate...
13. January 2016
The aim of this study was to formulate a microparticulate delivery system to deliver cinnarizine (CIN) directly to its site of absorption to overcome its low oral bioavailability. Enteric microparticles were prepared by varying ratios of pH-sensitive polymers (Eudragit L100 and Eudragit S100). A full 33 factorial experimental design was adopted to evaluate the effect of variables (CIN concentration as well as Eudragit’s concentration) on the tested parameters, namely, particle size (p.s.),...