- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. July 2018
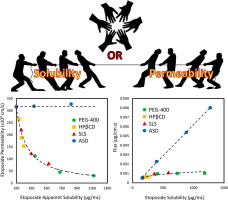
Poor aqueous solubility is a major challenge in today's biopharmaceutics. While solubility-enabling formulations can significantly increase the apparent solubility of the drug, the concomitant effect on the drug's apparent permeability has been largely overlooked. The mathematical equation to describe the membrane permeability of a drug comprises the membrane/aqueous partition coefficient, which in turn is dependent on the drug's apparent solubility in the GI milieu, suggesting that the...
04. September 2017
This work presents a review of literature and experimental data relevant to the possibility of waiving pharmacokinetic bioequivalence studies in human volunteers for approval of immediate release solid oral pharmaceutical forms containing folic acid as the single active pharmaceutical ingredient. For dosage forms containing 5 mg folic acid, the highest dose strength on the WHO Essential Medicines List, the dose/solubility ratio calculated from solubility studies was higher than 250 mL,...
17. July 2016
The currently available list of excipients in the market is unending. They include diverse classes of small molecules and macromolecules of natural or semi-synthetic origin, and they represent all states of matter—gases, liquids, semi-solids, and solids. Excipients are essential ingredients in drug products independent of the route of administration. New use(s) of an approved excipient or the development of novel excipients are driven by scientific and business decisions to address unmet...
29. May 2016
Abstract Excipients represent diverse classes of molecules, small molecules or macromolecules, with versatile structures within a given class and are from natural, semi-synthetic and synthetic sources. They are essential ingredients in drug products independently of the route of administration where both traditional and non-traditional uses are present. Beyond their traditional use as formulation and manufacturing aids, certain excipients exhibit biological effects and thus can be used either...
31. March 2016
Abstract We previously concluded that 12 common excipients need not be qualitatively the same and quantitatively very similar to reference for Biopharmaceutics Classification System–based biowaivers. This conclusion for regulatory relief is based upon a series of bioequivalence studies in humans involving cimetidine and acyclovir. Limitations were also discussed. We understand the major concern of García-Arieta et al. is that “results obtained by Vaithianathan et al. should not be...
31. March 2016
The objective was to assess the impact of larger than conventional amounts of 14 commonly used excipients on Biopharmaceutics Classification System (BCS) class 3 drug absorption in humans. Cimetidine and acyclovir were used as model class 3 drugs across three separate four-way crossover bioequivalence (BE) studies (n ¼ 24 each) in healthy human volunteers, denoted as study 1A, 1B, and 2. In study 1A and 1B, three capsule formulations of each drug were manufactured, collectively involving 14...
01. February 2016
Poor water solubility of many drugs has emerged as one of the major challenges in the pharmaceutical world. Polymer-based amorphous solid dispersions have been considered as the major advancement in overcoming limited aqueous solubility and oral absorption issues. The principle drawback of this approach is that they can lack necessary stability and revert to the crystalline form on storage. Significant upfront development is, therefore, required to generate stable amorphous formulations. A...
14. November 2015
Using partial least square discriminate analysis (PLSDA), we studied the spectroscopic differences between the commonly used filler‐binder microcrystalline cellulose (MCC) from five manufactures. These samples had subtle differences in the chemical and physical properties, which are often the cause of differences in excipient performance. Studying these differences allowed us to build and validate a model to classify five manufacturers of MCC using near-infrared (NIR) spectra. More