- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
29. April 2017
Abstract Thoughtfully designed early clinical formulations not only meet the needs of the study at hand and inform the development of the commercial product, but can influence the direction of the clinical program as well as provide further guidance to potential backups still in exploratory stages. This chapter focuses on the various types of early clinical formulations, why they are developed, and how the preclinical formulation space helps to guide initial clinical formulation selection....
30. March 2017
Abstract Albeit the rapidly evolving knowledge about tumor biochemistry enables various new drug molecules to be designed as treatments, malignant central nervous system (CNS) tumors remain untreatable due to the failure to expose the entire tumor to such therapeutics at pharmacologically meaningful quantities. Therefore, drug delivery in CNS tumors must be properly addressed, as otherwise, novel therapies will continue to fail. In this regard, nanomedicine poses an appealing platform for...
25. September 2016
Abstract Over the past two decades, there has been growing concern over the lack of proper medication for children. This review attempts to evaluate the current progress of EU Pediatric Regulation made since 2007. The lack of properly evaluated pediatric medication has for long been a source of concern in the European Union. The drugs that were used in the past were often not properly evaluated, and dosage was arbitrarily calculated. Therefore, it was necessary to establish the Pediatric...
09. August 2016
Abstract Direct delivery of sustained therapeutic levels of mesalamine (MS) via rectal systems to manage distal forms of ulcerative colitis was studied. The High molecular weight hydroxypropyl methylcellulose (HPMC K4M) polymer was combined with hydrophilic surfactants to control polymer hydration process allowing optimization of the mucoadhesive and controlled drug release properties for the rectal systems. Physical mixtures and granules of MS and HPMC K4M were prepared and in vitro...
21. June 2016
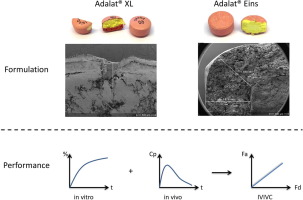
Aims Food intake is known to have various effects on gastrointestinal luminal conditions in terms of transit times, hydrodynamic forces and/or luminal fluid composition and can therefore affect the dissolution behavior of solid oral dosage forms. The aim of this study was to investigate and detect the dosage form-dependent food effect that has been observed for two extended-release formulations of nifedipine using in vitro dissolution tests. Methods Two monolithic extended release formulations,...
27. February 2016
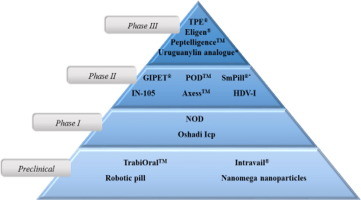
The development of oral dosage forms that allows absorption of therapeutic peptides to the systemic circulation is one of the greatest challenges for the pharmaceutical industry. Currently, a number of technologies including either mixtures of penetration enhancers or protease inhibitors and/or nanotechnology-based products are under clinical development. Typically, these formulations are presented in the form of enteric-coated tablets or capsules. Systems undergoing preclinical investigation...