- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
02. July 2018
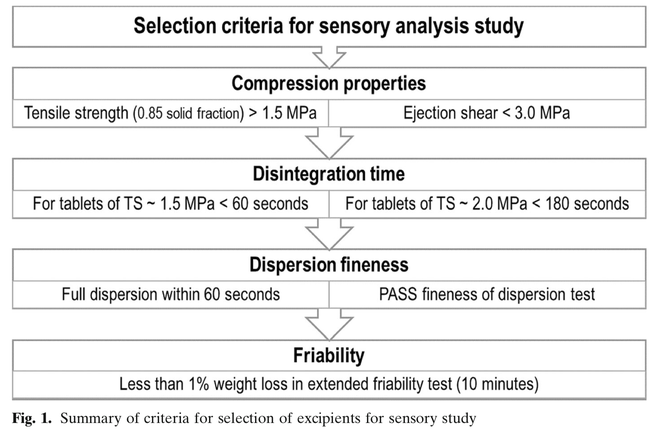
Palatability and patient acceptability are critical attributes of dispersible tablet formulation. Co-processed excipients could provide improved organoleptic profile due to rational choice of excipients and manufacturing techniques. The aim of this study was to identify the most suitable co-processed excipient to use within directly compressible dispersible tablet formulations. Nine excipients, selected based on successful manufacturability, were investigated in a randomised, preference and...
19. January 2016
This paper deals with a study of the novel coprocessed dry binder Combilac®, which contains 70% of α-lactose monohydrate, 20% of microcrystalline cellulose and 10% of native corn starch. These tests include flow properties, compressibility, lubricant sensitivity, tensile strength and disintegration time of tablets. Compressibility is evaluated by means of the energy profile of compression process, test of stress relaxation and tablet strength. More
13. May 2015
Meggle has launched the new high-functionality excipient (HFE) CombiLac, a lactose-based, co-processed excipient, specifically designed to ease oral solid dosage form development and manufacture in direct compression (DC). See more at: http://www.contractpharma.com/contents/view_breaking-news/2015-05-13/meggle-introduces-triple-compound-combilac/#sthash.eH27ZowB.dpuf
08. March 2015
Meggle Pharma: Soon we will introduce a world first: CombiLac® a triple compound which will ease formulations by combining diverging features. http://www.meggle-pharma.com/en/lactose/25-combilacsup-sup.html Does anybody know when it will be launched and more information is available?