- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
01. October 2018
In this study, a set of 24 experiments was designed to understand the combined effect of different process parameters, i.e. material feed rate, liquid-to-solid (L/S) ratio, screw speed, and screw configuration on the residence time distribution, granule morphology, and particle size distribution in twin screw wet granulation of microcrystalline cellulose. It was shown that residence times were longer at higher L/S and lower screw speeds. However, the most dominant effect on mean residence time...
20. August 2018
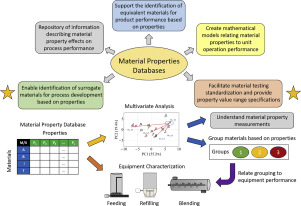
Material properties are known to have a significant impact on pharmaceutical manufacturing performance, particularly for solid product processes. Evaluating the performance of a specific material, for example an active pharmaceutical ingredient or excipient, is critical during development stages in order to determine the impact of material properties on the process. However, materials may be scarce during the early stages of process development due to high cost, unavailability, import...
20. August 2018
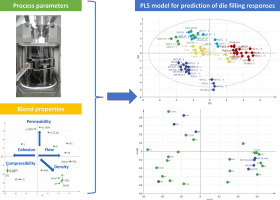
Based on characterization of a wide range of fillers and APIs, thirty divergent blends were composed and subsequently compressed on a rotary tablet press, varying paddle speed and turret speed. The tablet weight variability was determined of 20 grab samples consisting of each 20 tablets. Additionally, the bulk residence time, ejection force, pre-compression displacement, main compression force, die fill fraction and feed frame fill fraction were determined during each run. Multivariate data...
17. August 2018
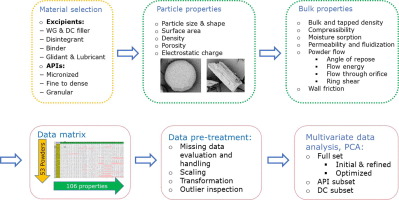
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
17. August 2018
In continuous manufacturing of solid dosage forms, continued assurance of process performance and product quality is based on accurate and consistent flow of solid materials. Acknowledging the multidimensionality of material flow properties is often the first step to explore the material knowledge space.
17. July 2018
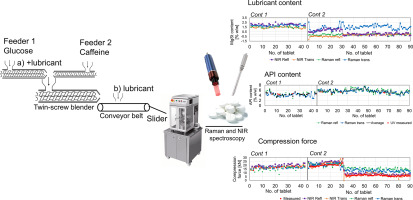
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
16. June 2018
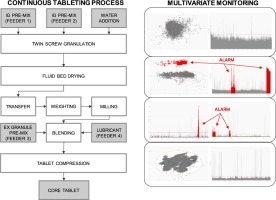
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of...
14. June 2018
The pharmaceutical industry has found new applications for the use of continuous processing for the manufacture of new therapies currently in development. The transformation has been encouraged by regulatory bodies as well as driven by cost reduction, decreased development cycles, access to new chemistries not practical in batch, improved safety, flexible manufacturing platforms, and improved product quality assurance. The transformation from batch to continuous manufacturing processing is the...
07. June 2018
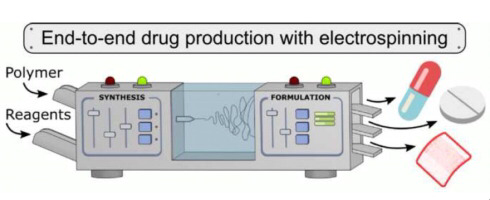
Based on the concept of continuous manufacturing an end-to-end benchtop device was developed unprecedented for the production of solid drug dosage forms by connecting flow synthesis and formulation via electrospinning (ES). Together with the optimized two-step continuous-flow synthesis of acetylsalicylic acid (ASA) a water-soluble polymeric excipient (polyvinylpyrrolidone K30, PVPK30) was introduced. The resulting polymeric solution could be readily electrospun into solid nanofibers with high...