- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
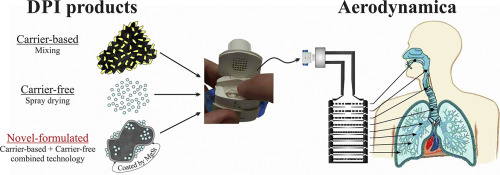
25. July 2018
Dry Powder Inhaler (DPI) could offer a propellant-free, easy-to-use powder form ensuring better stability than liquid dosae forms. Therefore the development of traditional carrier-based and carrier-free new generation systems is a determinative factor in the field of DPI formulation. The purpose of our research work was to combine these two systems, utilizing their beneficial properties to produce a novel pulmonary drug delivery system containing ciprofloxacin hydrochloride (CIP). Co-spray...
08. February 2018
Development of bi-component generic orally inhaled product (OIP) delivered from dry powder inhaler (DPI) is challenging due to the necessity to demonstrate the similarity of size distribution of both APIs in inhaled aerosol. The effects of selected technical factors on OIP development are investigated. The Monodose inhalers with different aerodynamic resistance were used to aerosolize generic fluticasone/salmeterol formulations. Flow dynamics in the DPIs was analyzed during realistic use. The...
06. November 2017
Many efforts have been made in the past to understand the function of lactose fines which are given as a ternary component to carrier-based dry powder inhaler formulations. It is undisputed that fines can significantly improve the performance of such formulations, but choosing the right amount of fines is a crucial point, because too high concentrations can have negative effects on the dispersion performance
17. October 2017
Dry powder inhaler for pulmonary drug delivery: human respiratory system, approved products and therapeutic equivalence guideline
16. October 2017
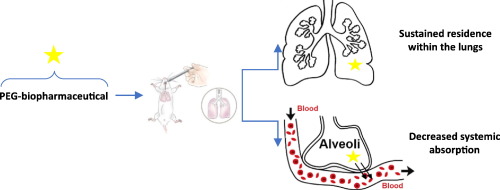
More and more therapeutic proteins are developed for an administration by inhalation to treat respiratory diseases. PEGylation is an interesting approach for sustaining the residence time of these biopharmaceuticals in the lungs and thereby decrease the frequency of administration and the daily burden of inhalation therapies.
21. March 2016
DFE Pharma is a leading specialist in production of excipients for virtually every kind of inhalation device and most inhaled treatments. Inhalation lactose Lactose is by far the most important carrier used for inhalants. It is one of the very few substances accepted by all medical and regulatory authorities as being ideal for inhaled medicines. Every type of inhaler requires a different grade and blend of lactose to ensure the best possible performance.DFE Pharma makes a comprehensive range of...
22. February 2016
The principle of carrier-based blends is well established in formulations for dry powder inhalation, as it overcomes problems of micronized drug particles often being cohesive and poorly flowable and hence, creating difficulties in powder handling and dosing.1 In carrier-based blends, micronized drug is adhered on the surface of the carrier and thus, is moved and dosed as if it were a larger particle. For nasal administration, particles larger than 10 μm, probably better than 50 μm, are...
27. October 2015
10. July 2015
This study aims to investigate the effect of carrier characteristics and dosator capsule filling operation on the in vitro deposition of mixtures containing salbutamol sulphate (SS) and lactose and mannitol as model carrier materials. The carrier surfaces of lactose and mannitol were modified via wet decantation. More