- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. February 2018
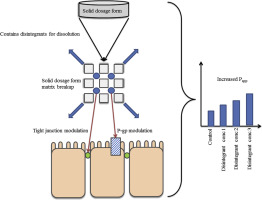
Abstract Pharmaceutical excipients were designed originally to be pharmacologically inert. However, certain excipients were found to have altering effects on drug pharmacodynamics and/or pharmacokinetics. Pharmacokinetic interactions may be caused by modulation of efflux transporter proteins, intercellular tight junctions and/or metabolic enzyme amongst others. In this study, five disintegrants from different chemical classes were evaluated for P-glycoprotein (P-gp) related inhibition and tight...
08. January 2017
ABSTRACT: The aim of the present work was to prepare and evaluate fast dissolving tablets of nebivolol with a view to enhance patient compliance and minimize the side effects. In this study, fast dissolving tablets of nebivolol were formulated by direct compression method using mucilages of tapioca seeds (Manihot esculenta), basella climb (Basella alba), red sorrel (Hibiscus sabdariffa) as natural disintegrants and crosspovidone as a synthetic superdisintegrant in different ratios with directly...
16. February 2016
Disintegrant is one of the most important components in a typical tablet dosage form. It is responsible for ensuring the break-up of the tablet matrix upon ingestion. Disintegrants act by different mechanisms, and a number of factors may affect their performance. It is important for formulators to understand how disintegrants function so as to be able to judiciously use disintegrants to develop optimized formulations. If the formulator is required to implement the quality by design paradigm...
10. November 2015
To ensure safe oral administration, pediatric patients require an appropriate dosage form to be swallowed without relevant difficulties. Ex tempore hydrated powders, forming viscous pulp “on a spoon”, have recently gained much interest as pediatric formulations. The aim of this study was to evaluate the viscosity-increasing substances and disintegrants, alone or in mixtures, as excipients suitable for preparing such formulations, with candesartan and valsartan chosen as model active...