- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. September 2018
Orodispersible films (ODFs) provide high application comfort due to rapid disintegration in the oral cavity. They increasingly found the approval of pharmaceutical research and development and were added to the European Pharmacopeia in 2012. The European Pharmacopeia explicitly demands disintegration testing for ODFs, but does not refer to a suitable method. The aim of this study was to collect and evaluate existing disintegration methods regarding their suitability to investigate different ODF...
10. September 2018
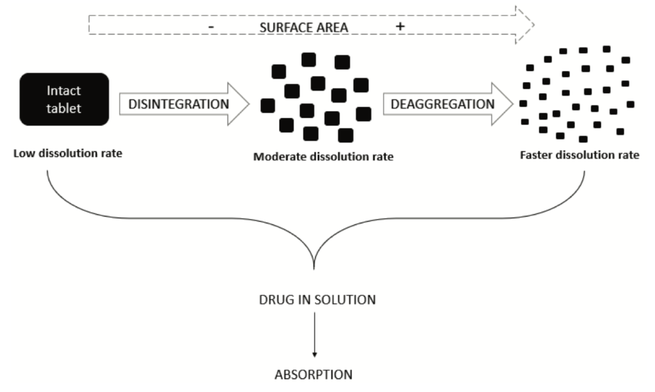
Disintegration is a physical process related to the mechanical breakdown of a tablet or granulate particle into smaller particles. This review investigates disintegration mechanisms, pharmacopeial use of the disintegration test and scientific studies showing its utility and potential as a pharmaceutical performance test. With a proper dosage form understanding and demonstration/justification of the mechanistic details of drug dissolution from a dosage form, dissolution testing might be replaced...
24. June 2018
Co-processed excipients may enhance functionality and reduce drawbacks of traditional excipients for the manufacture of tablets on a commercial scale. The following study aimed to characterise a range of co-processed excipients that may prove suitable for dispersible tablet formulations prepared by direct compression. Co-processed excipients were lubricated and compressed into 10.5-mm convex tablets using a Phoenix compaction simulator. Compression profiles were generated by varying the...
17. May 2018
Current in vitro disintegration methods for polymeric films are qualitative and introduce significant user bias. The goal of these studies is to develop a novel, quantitative disintegration technique which can be used to characterize polymeric films in vitro. Methods A method was developed using a Texture Analyzer instrument to evaluate film disintegration. Solvent-casted, clinically advanced, anti-HIV, vaginal films as well as marketed vaginal films were used throughout these studies. Method...
08. May 2018
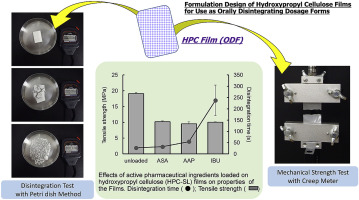
Hydroxypropyl cellulose (HPC) is a water-soluble polymer used as a binder during pharmaceutical tableting and granulation. HPC is also known as a base material for pharmaceutical film by virtue of its film formability with excellent plasticity. The aim of this study was to assess the applicability of HPC to orally disintegrating film (ODF) and to investigate optimization of the ODF formulation of HPC. The effects of the molecular weight of HPC and the addition of active pharmaceutical...
26. February 2018
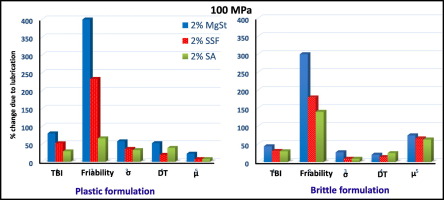
As an essential formulation component for large-scale tablet manufacturing, the lubricant preserves tooling by reducing die-wall friction. Unfortunately, lubrication also often results in adverse effects on tablet characteristics, such as prolonged disintegration, slowed dissolution, and reduced mechanical strength. Therefore, the choice of lubricant and its optimal concentration in a tablet formulation is a critical decision in tablet formulation development to attain low die-wall friction...
13. February 2018
As an essential formulation component for large-scale tablet manufacturing, the lubricant preserves tooling by reducing die-wall friction. Unfortunately, lubrication also often results in adverse effects on tablet characteristics, such as prolonged disintegration, slowed dissolution, and reduced mechanical strength.
18. December 2017
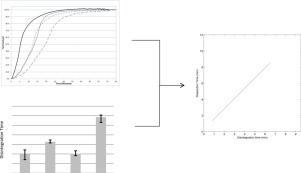
The drug release rate of a rapidly dissolving immediate-release tablet formulation with a highly soluble drug is proposed to be controlled by the disintegration rate of the tablet. Disintegration and dissolution test methods used to evaluate the tablets were shown to discriminate manufacturing process differences and compositionally variant tablets.
18. November 2017
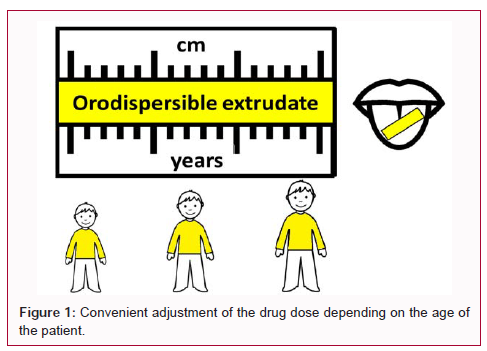
The aim of the present investigation is to produce rapidly disintegrating laminar extrudates for delivering ibuprofen in the mouth of paediatric patients. The laminar shape is particularly convenient for drug delivering in the mouth and can be easily cut in different sizes allowing for a convenient adjustment of the drug dose depending on the age of the patient. Due to the fact that in paediatric formulations, the selection of the excipients is always a challenging issue and the reduction of...
21. October 2017
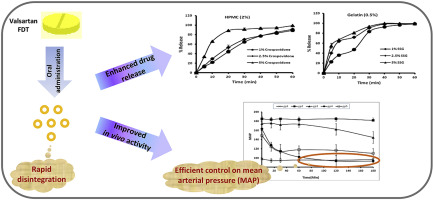
Fast disintegrating tablets (FDTs) dissolve or disintegrate in the mouth without the need of additional water. The aim of the present study was to formulate FDTs of Valsartan for the treatment of hypertension in children who could find difficulties in swallowing conventional solid dosage forms. The tablets were prepared by wet granulation technique. Superdisintegrants such as sodium starch glycolate (SSG) and crospovidone were optimized as 5% on the basis of least disintegration time. Different...