- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
12. September 2018
The amorphous solid dispersion (ASD) technique has been employed to formulate poorly-soluble drugs, however, development of solid dosage forms with ASD is challenging due to the high propensity of amorphous drug to precipitate upon dissolution. Thus this work aimed to explore the potential of controlled release amorphous solid dispersion (CRASD) systems using polyvinyl acetate (PVAc) as a release-retarding excipient to mitigate the drug precipitation during dissolution of poorly water-soluble...
30. August 2018
Hard gelatin capsule (HGC) shells are widely used to encapsulate drugs for oral delivery, but are vulnerable to gelatin cross-linking, which can lead to slower and more variable in vitro dissolution rates. Adding proteolytic enzymes to the dissolution medium can attenuate these problems, but this complicates dissolution testing and is only permitted by some regulatory authorities. Here, we expand the scope of our previous work to demonstrate that canisters containing activated carbon (AC) or...
17. August 2018
In continuous manufacturing of solid dosage forms, continued assurance of process performance and product quality is based on accurate and consistent flow of solid materials. Acknowledging the multidimensionality of material flow properties is often the first step to explore the material knowledge space.
04. July 2018
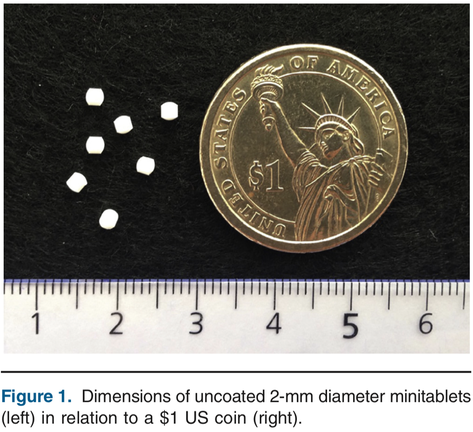
The objective of this study was to assess the acceptability and swallowability of several minitablets when administered as a unit dose compared with an equivalent dose of syrup in children aged 6 months to 5 years. Study design The acceptability and swallowability of multiple drug-free minitablets in comparison with glucose syrup was assessed in 372 children of 2 age groups (186 in age group 1 [6-23 months of age] and 186 in age group 2 [2-5 years of age]) in a randomized, 3-way, single...
28. May 2018
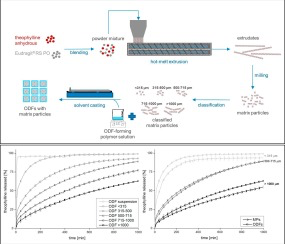
Orodispersible films (ODFs) are an advantageous dosage form to accomplish patient convenience and compliance in oral drug delivery. They provide a number of special application features, such as the ease of administration without water and suitability for patients with swallowing problems. However, this promising dosage form has been limited to immediate release formulations so far. The aim of this study was to develop a thin film produced by solvent casting, which is rapidly disintegrating...
29. March 2018
Docetaxel (DTX) is a chemotherapy drug that can be used for different type of cancers. Due to polysorbate 80, the excipient in the formulation, acute hypertensivity reaction is observed after intravenous administration. The development of oral formulation for DTX has always been problematic as the bioavailability of the drug is shown to be low due to P-glycoprotein efflux transporters and the intestinal metabolism by CYP3A4 enzymes. The objective of this study is to develop novel DTX in situ...
24. February 2018
Enalapril is an off-patent angiotensin-converting enzyme inhibitor for which no paediatric age-appropriate formulation is commercially available in Europe, and enalapril maleate (EM) orodispersible minitablets (ODMTs) have previously been formulated within the LENA (labelling enalapril from neonates to adolescents) project. In this study, a dilution method has been developed by dispersing the lowest dose strength ODMTs to enable flexible and precise EM dosing during the dose titration phase of...
08. November 2017
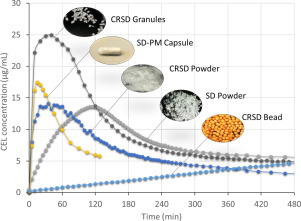
Colon delivery systems for oral administration have grown in popularity since the 1990s, primarily because of the increasing incidence of inflammatory bowel disease (IBD) that has broadly been demonstrated to benefit from topical pharma- cological treatment.
18. October 2017
According to pediatric experts’ recommendations, an appropriate formulation for children allows minimal dosage and frequency; has minimal impact on lifestyle; contains non-toxic excipients; enables convenient, easy and reliable administration; and is easily produced and commercially viable.
04. October 2017
A Capsugel paper on the search for physiologically unobjectionable coloring agents for pharmaceutical drug formulations in capsules. Comparative analysis between the caplet and capsules where capsules are shown to shorten development times. Download