- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. June 2018
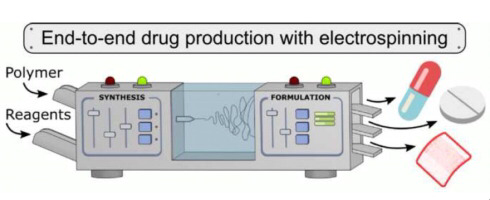
Based on the concept of continuous manufacturing an end-to-end benchtop device was developed unprecedented for the production of solid drug dosage forms by connecting flow synthesis and formulation via electrospinning (ES). Together with the optimized two-step continuous-flow synthesis of acetylsalicylic acid (ASA) a water-soluble polymeric excipient (polyvinylpyrrolidone K30, PVPK30) was introduced. The resulting polymeric solution could be readily electrospun into solid nanofibers with high...
29. May 2018
Powder flow is critical to the success of various pharmaceutical processes such as tableting and capsule filling. Despite a plethora of flow characterisation techniques and parameters available, powder flow still remains to be a not well understood subject. Inter-relationships between the various powder flow parameters in particular have not been well established. Furthermore, while it is known that particle size and shape are important determinants of powder flow, their relative impact on...
27. May 2018
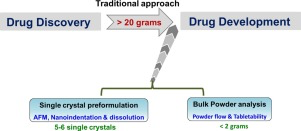
A systematic assessment of key material attributes of active pharmaceutical ingredients (APIs) using minimal material approach is illustrated at the particulate level and bulk level. At a bulk level, flowability improvement of an API through crystal habit manipulation is exemplified. The impact of crystal structures and particulate properties (crystal habit and crystal size) on their mechanical behaviour were addressed by measuring powder tabletability. Bulk powder flow and tabletability were...
02. May 2018
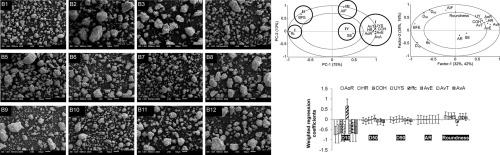
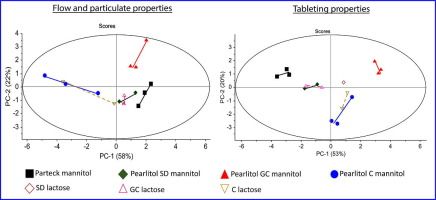
Appropriate selection of excipient grade during tablet formulation development depends on thorough knowledge in their compaction and flow properties. Each chemically unique pharmaceutical excipientis usually available in several commercial grades that are widely different in powder properties, which influence their performance for a specific formulation application. In this work, 11 grades of mannitol were systematically characterized, in terms of their particulate, flow and tableting...
01. May 2018
Downstream processing aspects of a stable form of amorphous itraconazole exhibiting enhanced dissolution properties were studied. Preparation of this ternary amorphous solid dispersion by either spray drying or hot melt extrusion led to significantly different powder processing properties. Particle size and morphology was analysed using scanning electron microscopy. Flow, compression, blending and dissolution were studied using rheometry, compaction simulation and a dissolution kit. The spray...
06. November 2017
Many efforts have been made in the past to understand the function of lactose fines which are given as a ternary component to carrier-based dry powder inhaler formulations. It is undisputed that fines can significantly improve the performance of such formulations, but choosing the right amount of fines is a crucial point, because too high concentrations can have negative effects on the dispersion performance
20. April 2017
Abstract The purpose of this study is to demonstrate that the flow function (FFc) of pharmaceutical powders, as measured by rotational shear cell, is predominantly governed by cohesion, but not friction coefficients. Driven by an earlier report showing an inverse correlation between FFc and the cohesion divided by the corresponding pre-consolidation stress [Wang et al. 2016. Powder Tech. 294:105-112], we performed analysis on a large dataset containing 1130 measurements from a ring shear...
03. January 2017
Abstract Understanding interparticle interactions in powder systems is crucial to pharmaceutical powder processing. Nevertheless, there remains a great challenge in identifying the key factors affecting interparticle interactions. Factors affecting interparticle interactions can be classified in three different broad categories: powder properties, environmental conditions, and powder processing methods and parameters. Although, each of these three categories listed is known to affect...
14. December 2016
Abstract The purpose of the study was to develop and evaluate a tripartite novel excipient for direct compression of salbutamol tablets. Various batches (A-E) of the novel excipient was prepared by co-processing varying concentrations of okro gum with gelatinized maize starch and lactose using co-precipitation method. The novel excipient powders were subjected to some physicochemical evaluations. Batches (F-H) of the physical mixture of the novel excipient ingredients were also prepared. The...
15. August 2016
Abstract The methods used for flow characterization of a powder mass include the angle of repose (AOR), Carr index (CI), and powder flow tester (PFT). The use of nanosilica as a flow modifier (glidant) is very common in industry. This study aims to compare the glidant effect of hydrophobic and hydrophilic silica on a poorly flowable active pharmaceutical ingredient (ibuprofen) by different flow characterization techniques. Different percentages (0.5, 1.0, and 2.0 wt%) of both types of mixed...