- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. September 2018
Usage of the amorphous phase of compounds has become the method of choice to overcome oral bioavailability problems related to poor solubility. Due to the unstable nature of glasses, it is clear that the method preparation of the amorphous glass will have an impact on physical/chemical stability and in turn in vivo performance. The method of preparation can also have a profound impact on the mechanical properties of the amorphous phase. We have explored the impact of preparation method on the...
13. August 2018
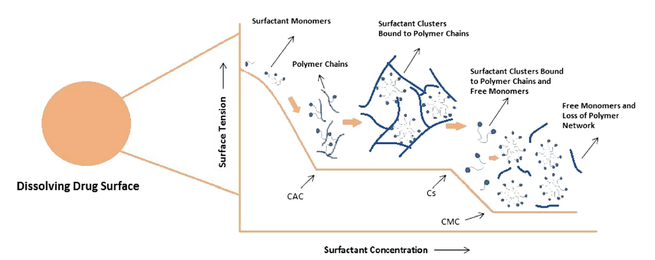
Surfactants are commonly incorporated in conventional and enabled formulations to enhance the rate and extent of dissolution of drugs exhibiting poor aqueous solubility. Generally the interactions between the drug and excipients are systematically evaluated, however, limited attention is paid towards understanding the effect of interaction between functional excipients and its impact on the performance of the product. In the current study, the effect of potential interaction between a nonionic...
13. August 2018
The aim of this study is to determine favorable process conditions for the coating of placebo tablets. Tablets made of microcrystalline cellulose are coated with hydroxypropyl cellulose polymer and Advantia™ Prime polymeric mixture film in lab-scale fluid-bed environment with a Wurster tube. In order to determine favorable process conditions (concentration, Wurster tube position, inlet air temperature, and atomization pressure), evaluation factors expressing process efficiency were...
08. August 2018
Combinatorial chemistry, computational molecular modeling and high throughput screening in drug discovery have significantly increased the number of poorly soluble drugs. About 40% of drugs developed in the past and about 90% of the drugs in development are poorly soluble drugs. When administered orally, a drug has to first dissolve in gastrointestinal fluids before it can be absorbed in to the blood and reach its site of action. The objective of this review article is to outline the key...
20. June 2018
Itraconazole is a fungicide drug which has low bioavailability due to its poor water solubility. Amorphous solid dispersion (ASD) is a tool that has the potential to greatly increase the dissolution rate and extent of compounds. In this work, the dissolution of tablets containing the ASD of itraconazole with either hydroxypropyl methylcellulose (HPMC) or vinylpyrrolidone-vinyl acetate copolymer (PVPVA) was compared in order to find a formulation which can prevent the drug from the precipitation...
07. June 2018
Wet granulation is mostly used process for manufacturing matrix tablets. Compared to the direct compression method, it allows for a better flow and compressibility properties of compression mixtures. Granulation, including process parameters and tableting, can influence critical quality attributes (CQAs) of hydrophilic matrix tablets. One of the most important CQAs is the drug release profile. We studied the influence of granulation process parameters (type of nozzle and water quantity used as...
06. May 2018
In the course of application and modernization of buccal dosage forms, lyophilized sponges for transmucosal drug delivery symbolize one of the most attractive approaches. Chitosan (CS) has been extensively investigated as a forming material of different buccal dosage forms including sponges. However, CS-based buccal delivery systems suffer from many limitations like weak adhesion strength and poor tensile properties. So, for the first time, the current study focused on the polymer blending...
05. May 2018
Along with the development of novel drug delivery systems the material science is also advancing. Conventional and novel synthetic or natural excipients provide opportunities to design dosage forms of the required features including their bioavailability.
24. April 2018
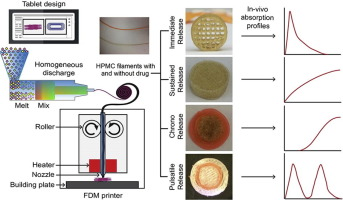
Three-dimensional printing (3DP), though developed for nonmedical applications and once regarded as futuristic only, has recently been deployed for the fabrication of pharmaceutical products. However, the existing feeding materials (inks and filaments) that are used for printing drug products have various shortcomings, including the lack of biocompatibility, inadequate extrudability and printability, poor drug loading, and instability. Here, we have sought to develop a filament using a single...
24. April 2018
Although strip films are a promising platform for delivery of poorly water-soluble drug particles via slurry casting, the effect of critical materials attributes (CMAs), e.g., super-disintegrants on critical quality attributes (CQAs), including film disintegration time (DT), remains under-explored. A two-level factorial design is considered to examine the impact of the super-disintegrant (SDI) type (sodium starch glycolate and croscarmellose sodium), their amount, and film thickness. SDIs were...