- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. February 2018
The poor oral bioavailability of many active pharmaceutical ingredients (APIs) resulting from low solubility is one of the important challenges in pharmaceutical technology. Over the last two decades the number of relatively insoluble drugs has grown steadily. Nowadays it is estimated that approximately 70% of new drug candidates are characterized by poor solubility.
26. January 2018
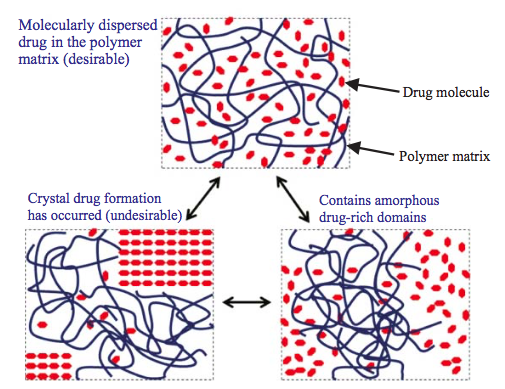
The understanding of amorphous solid dispersions has grown significantly in the past decade. This is evident from the number of approved commercial amorphous solid dispersion products. While amorphous formulation is considered an enabling technology, it has become the norm for formulating poorly soluble compounds.
20. January 2018
A solid dispersion is a dosage form in which an active ingredient (a drug) is mixed with at least one inert solid component. The purpose of the inert component is usually to improve the bioavailability of the drug. In particular, the inert component is frequently chosen to improve the dissolution rate of a drug that is poorly soluble in water.
11. January 2018
The oral bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs) can be improved by the preparation of amorphous solid dispersions (ASDs) where the API is dissolved in polymeric excipients. Desired properties of such ASDs like storage stability, dissolution behavior, and processability can be optimized by additional excipients. In this work, the influence of so-called low-molecular-weight excipients (LMWEs) on the phase behavior of ASDs was investigated.
24. December 2017
The preparation of amorphous solid dispersions (ASDs) is a well-established strategy for formulating active pharmaceutical ingredients by embedding them in excipients, usually amorphous polymers. Different polymers can be combined for designing ASDs with desired properties like an optimized dissolution behavior.
14. December 2017
Dow Pharma Solutions demonstrated its commitment to advancing the pharmaceutical sciences by exhibiting research findings in a range of posters at the 2017 AAPS show in San Diego, CA. Please find below links to copies of our presented posters.
04. December 2017
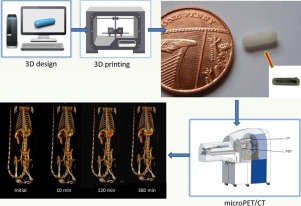
Fused deposition modelling (FDM) 3D printing (3DP) is a revolutionary technology with the potential to transform drug product design in both the pre-clinical and clinical arena. The objective of this pilot study was to explore the intestinal behaviour of four different polymer-based devices fabricated using FDM 3DP technology in rats.
27. October 2017
The primary aim of this study was to identify pharmaceutically acceptable amorphous polymers for producing 3D printed tablets of a model drug, haloperidol, for rapid release by fused deposition modeling (FDM).
12. October 2017
Solid dispersion formulation is a promising method to maintain in vivo drug solubility and to improve drug efficacy. However, the exact drug stabilization and release mechanisms of the solid dispersion formulation are unclear.
06. December 2015
Amorphous solid dispersions (ASDs) are of great interest as enabling formulations because of their ability to increase the bioavailability of poorly soluble drugs. However, the dissolution of these formulations under non-sink dissolution conditions results in highly supersaturated drug solutions that can undergo different types of phase transitions. More