- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. September 2018
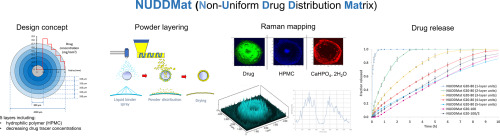
A decrease in the release rate over time is typically encountered when dealing with hydrophilic matrix systems for oral prolonged release due to progressive increase of the distance the drugmolecules have to cover to diffuse outwards and reduction of the area of the glassy matrix at the swelling front. In order to solve this issue, a novel formulation approach based on non-uniformdistribution of the active ingredient throughout the swellable polymer matrix was proposed and evaluated. Various...
07. May 2018
The aim of this research was to design and evaluate a hydrophilic matrix system for sustained release of glipizide, a weakly acidic poor soluble drug. A combination of inclusion complexation and microenvironmental pH modification techniques was utilized to improve the dissolution and pH-independent release of glipizide. Hydroxypropyl-β-cyclodextrin (HP-β-CD) was used as the complexation agent while sodium citrate and magnesium oxide (MgO) were used as model pH modifiers. The hydrophilic...
22. November 2017
Lipid nanoparticles are widely explored in many therapeutic, targeting, and imaging areas. Among the various nanoplatforms, lipid-based nanostructures offer better attributes to drug delivery, such as high loading capacity, biocompatibility, improved physicochemical and long-term stability, ease of surface modification, adjustable drug release, and enhanced bioavailability.
22. March 2017
Abstract The purpose of this study is to characterize controlled release matrix tablets of captopril and to find out the physicochemical properties that have an effect on the mucoadhesion process. The hydrophilic matrix tablets contain captopril, microcrystalline cellulose, barium sulfate, ascorbic acid, ethylcellulose N100, hydroxypropylmethylcellulose K15M, talc, magnesium stearate and colloidal silicon dioxide. The physicochemical properties of the formulations have been characterized using...
21. February 2017
Abstract: The aim of this work was to develop cefdinir solid dispersions (CSDs) prepared using hydrophilic polymers with enhanced dissolution/solubility and in vivo oral bioavailability. CSDs were prepared with hydrophilic polymers such as hydroxypropyl-methylcellulose (HPMC; CSD1), carboxymethylcellulose-Na (CMC-Na; CSD2), polyvinyl pyrrolidone K30 (PVP K30; CSD3) at the weight ratio of 1:1 (drug:polymer) using a spray-drying method. The prepared CSDs were characterized by aqueous solubility,...
20. February 2017
The main objective of the present study was to apply QbD methodology in the development of once-a-day sustained release quetiapine tablets. The quality target product profile (QTPP) was defined after the pharmaceutical properties and kinetic release of the innovator product, Seroquel XR 200 mg. For the D-optimal experimental design, the level and ratio of matrix forming agents and the type of extra granular diluent were chosen as independent inputs, which represented critical formulation...
04. January 2017
Abstract In this study a protocol exploiting the combination of the ultrasonic atomization and the complexation between polyelectrolytes was developed to efficiently encapsulate a hydrophilic chemotherapeutic agent essentially used in the treatment of colon cancer, 5-fluorouracil, in enteric shell-core alginate-based microcarriers. The atomization assisted by ultrasound allowed to obtain small droplets by supplying low energy and avoiding drug degradation. In particular microcarriers were...
18. August 2016
Abstract This paper addressed the application of hydroxyethyl pachyman (HEP) as a novel matrix for sustained – release tablets, using diclofenac sodium (DS) as a model drug. The studies showed the HEP tablets prepared by wet granulation had much slower drug release as compared to those prepared by direct compression. Meanwhile, increasing the percentage of HEP in the formulations caused a decrease in drug release rates. Moreover, DS release from the HEP tablets was much higher at high pH...
21. March 2016
Abstract. The crystal structures of active pharmaceutical ingredients and excipients should be strictly controlled because they influence pharmaceutical properties of products which cause the change in the quality or the bioavailability of the products. In this study, we investigated the effects of microcrystalline cellulose (MCC) crystallinity on the hydrophilic properties of tablets and the hydrolysis of active pharmaceutical ingredient, acetylsalicylic acid (ASA), inside tablets by using...
09. February 2016
The purpose of this study was to investigate the influence of different hydrophilic polymers on the swelling, bioadhesion and mechanical strength of hydrocolloid wound dressings (HCDs) in order to provide an appropriate composition for a hydrocolloid wound dressing system. In this study, the HCDs were prepared with styrene-isoprene-styrene copolymer (SIS) and polyisobutylene (PIB) as the base using a hot melting method. Additionally, numerous SIS/PIB-based HCDs were prepared with six...