- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
22. August 2018
IPEC-Americas presents the Technically Unavoidable Particles in Excipients (TUPs) webinar on September 12, 11 a.m. to 1 p.m. (EDT) The webinar will discuss the relevance of TUPs in excipient products. The presenter, Ann Van Meter, will highlight the IPEC-Americas Technically Unavoidable Particle Profile Guide (TUPPs Guide), explain a risk-based approach for evaluating visible particles in excipients and encourage suitable diaglogue between excipient manufacturers with their pharmaceutical...
17. July 2018
Excipient World - IPEC-Americas premier excipient event - wants YOUR proposals and ideas. This conference is the ONLY U.S. event focused exclusively on excipients, showcasing formulation and delivery system advances, regulatory and compliance initiatives, and innovative product applications for pharmaceutical, biologic, veterinary medicine, and medical device / combination and emerging sector manufacturers that rely on excipients.
13. July 2018
Historically, excipient manufacturers have relied on the International Pharmaceutical Excipients Council (IPEC) and Pharmaceutical Quality Group's (PQG's) Joint IPEC-PQG Good Manufacturing Practices (GMP) Guide for Pharmaceutical Excipients [1]. The two organizations issued this guidance in 2006, and it was the product of a significant amount of work by IPEC's members. It provides a great baseline; the industry, however, has moved on.
25. June 2018
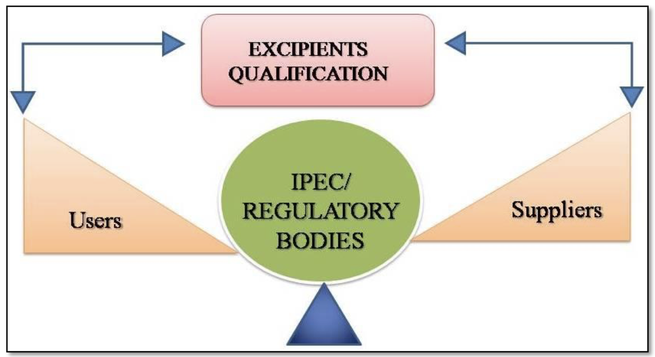
Excipients are an inactive substances formulated along with the active pharmaceutical ingredient(s) (APIs) of medication for bulking up of formulations or to give a therapeutic enhancement of API in the final dosage form. Almost of all the marketed products contain excipients thereby the excipients play a crucial role in the manufacture of medications by helping to preserve the efficacy, safety and functionality of the APIs. Excipients categorized as compendial excipients (official in...
30. May 2018
18-19 September 2018 in Cologne - Germany OBJECTIVES Implementation of GMP in an excipient manufacturing site Qualification and auditing of suppliers Analytical data and certificates of analysis for excipients The European Pharmacopoeia’s General Methods Modernisation Programme USP – Strategies and Opportunities for Excipient Standard Setting Particulate matter in pharmaceutical starting materials and drug products Regulatory and technical challenges for excipients in parenteral...
10. April 2018
Making Pharmaceuticals is covering the hot topics in the pharmaceutical industry from continuous manufacturing, supply chain, serialization, data integrity, regulatory, Brexit and many more in a 2 day conference and exhibition. Pharmaceutical excipient topics are covered in different sessions with the following presentations: Expanded Design Space in Hot Melt Extrusion with High Productivity Hypromellose Acetate Succinate Phospholipids as Business Opportunities for Pharmaceutical Line Extension...
23. March 2018
IPEC Federation and PDA will publish a technical report in 2019 to provide drugmakers guidelines on how to implement risk assessment challenges of excipient supply. Full Article
06. February 2018
The conference program of Making Pharmaceuticals Europe also cover important excipient topics with expert presentations on:
New IPEC Guidelines
Excipient Functionality Related Characteristics
Novel Excipients
30. January 2018
Arlington, VA —ExcipientFest will return to Puerto Rico to explore how crisis can be transformed into opportunity. The event will take place at the San Juan Marriott Stellaris Hotel on April 30th and May 1-2, 2018.
19. January 2018
IPEC Webinar - The concepts of what should be included in Quality Agreements have been explained in the 2009 IPEC Quality Agreement Guide and Templates. However, with new regulatory requirements and more complex supply chains, the Guide was recently revised by a team of IPEC experts to provide practical and detailed guidance on how to meet current challenges.