- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. August 2018
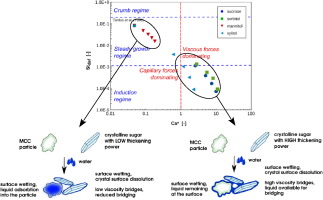
Sugars and sweeteners are common ingredients in foods and also in pharmaceutical industries. They are soluble and sticky ingredients and their processing in granulators may be difficult since they can easily adhere to mixer walls or leadto uncontrolled granule growth. The purpose of this research was to evaluate the feasibility of the high shear wet granulation process and to find the optimal amount of binder by studying the granulation performances of four sugars: mannitol, sorbitol, xylitol...
18. October 2017
According to pediatric experts’ recommendations, an appropriate formulation for children allows minimal dosage and frequency; has minimal impact on lifestyle; contains non-toxic excipients; enables convenient, easy and reliable administration; and is easily produced and commercially viable.
06. September 2017
Abstract: Liquid crystal (LC)-forming lipids represent an important class of biocompatible amphiphiles and their application extends to cosmeceutical, dietary, and pharmaceutical technologies. In the present study, we aimed to develop strategies for designing and optimizing oral and topical LC formulations by evaluating their in vitro and in vivo drug absorption performances. C17-Monoglycerol ester (MGE) was used as a LC-forming lipid. p-Amino benzoic acid, methyl PABA, ethyl PABA, and sodium...
12. April 2017
Abstract A new institutional clinical trial assessed the improvement of sleep disorders in 40 children with autism treated by immediate release melatonin formulation in different regimens (0.5 mg, 2 mg, and 6 mg daily) for one month. The objectives of present study were to (i) prepare low dose melatonin hard capsules for pediatric use controlled by two complementary methods and (ii) carry out a stability study in order to determine an use-by-date. Validation of preparation process was claimed...
26. January 2017
Abstract The interest of consumers and government organizations on improving the quality of food and its impact on human health is growing in recent years. Consuming low-calorie foodstuffs are increasingly demanded, and the presence of artificial sweeteners plays an important role not only in food but also in waste reaching the environment. Analysis of these compounds is important in assessing food safety and quality. This chapter reviews the analytical approaches for the extraction and...
20. August 2016
Objectives The aim of this review was to map the currently available evidence on acceptability of oral paediatric medicines to aid in the selection of suitable platform formulations for the development of new acceptable paediatric products. Methods This process used a defined search strategy of indexed publications and included methods to assess the quality of the evidence retrieved. Key findings Taste/palatability was the most extensively studied area of paediatric medicine acceptability yet...
20. June 2016
AIM It is the aim of this study to synthesize hyaluronic acid (HA) derivatives bearing mucoadhesive properties and showing prolonged stability at pH 7.4 and under oxidative condition as liquid dosage form. METHODS HA was modified by thiolation with L-cysteine (HA-SH) and by conjugation with 2-mercaptonicotinic acid-L-cysteine ligand to obtain an S-protected derivative (HA-MNA). The polymers were characterized by determination of thiol group content and mercaptonicotinic acid content....
26. February 2016
Excipients are essential for solubility enhancing formulations. Hence it is important to understand how additives impact key solution properties, particularly when supersaturated solutions are generated by dissolution of the solubility enhancing formulation. Herein, the impact of different concentrations of dissolved polymers on the thermodynamic and kinetic properties of supersaturated solutions of danazol were investigated. More
23. June 2015
A novel tablet product that in an easy, flexible and reproducible manner can be loaded with a relatively high amount of a pharmaceutically acceptable liquid formulation e.g. carrying a therapeutically, prophylactically and/or diagnostically active substance. The novel loadable tablet product may be produced in large-scale batches and stored until use and each batch or sub-batch may be loaded with the same or different pharmaceutically acceptable liquid formulations and/or active substances. A...