- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. August 2018
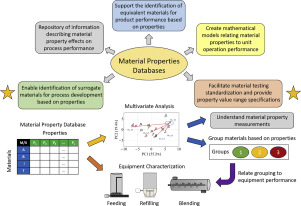
Material properties are known to have a significant impact on pharmaceutical manufacturing performance, particularly for solid product processes. Evaluating the performance of a specific material, for example an active pharmaceutical ingredient or excipient, is critical during development stages in order to determine the impact of material properties on the process. However, materials may be scarce during the early stages of process development due to high cost, unavailability, import...
17. August 2018
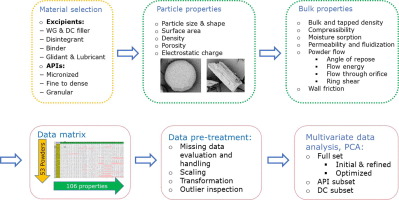
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
19. April 2018
Excipients are materials added along with therapeutic agents; and incompatibility between excipients and therapeutic agents affect the final outcome of the product. Aspirin is an ester and is prone to hydrolysis. This study focus on the effect of magnesium stearate as an excipient on stability of aspirin in granules. For that an accelerated degradation study was done on aspirin-magnesium stearate containing granules. For that, aspirin containing granules were sequentially mixed with increasing...
05. March 2018
This study presents a framework for process and product development on a continuous direct compression manufacturing platform. A challenging sustained release formulation with high content of a poorly flowing low density drug was selected. Two HPMC grades were evaluated as matrix former: standard Methocel CR and directly compressible Methocel DC2. The feeding behavior of each formulation component was investigated by deriving feed factor profiles.
21. April 2017
Abstract The potential of fine excipient materials to improve the performance of carrier-based dry powder inhalation mixtures is well acknowledged. The mechanisms underlying this potential are, however, open to question till date. Elaborate understanding of these mechanisms is a requisite for rational rather than empirical development of ternary dry powder inhalation mixtures. While effects of fine excipient materials on drug adhesion to and detachment from surfaces of carrier particle have...
11. January 2017
Abstract With the implementation of quality by design (QbD), critical attributes of raw material (drug substance and excipients) are of significantly importance in pharmaceutical manufacturing process. It is desirable for the quality control of critical material attributes (CMAs) of excipients to ensure the quality of end product. This paper explored the feasibility of an at-line method for the quantitative analysis of hydroxypropoxy group in hydroxypropyl methylcellulose (HPMC) with near...
17. November 2016
Abstract Control of drug action through formulation is a vital and very challenging topic within pharmaceutical sciences. Cellulose nanofibers (CNF) are an excipient candidate in pharmaceutical formulations that could be used to easily optimize drug delivery rates. CNF has interesting physico-chemical properties that, when combined with surfactants, can be used to create very stable air bubbles and dry foams. Utilizing this inherent property, it is possible to modify the release kinetics of the...
10. August 2016
Abstract The objective of this study was to analyze differences in the subtle microstructure of three different grades of HMPC hard capsule shells using mechanical, spectroscopic, microscopic and tomographic approaches. Dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), vibrational spectroscopic, X-Ray scattering techniques as well as environmental scanning electron microscopy (ESEM) and optical coherence tomography (OCT) were used. Two HPMC capsules manufactured via chemical...
04. July 2016
Abstract We aimed to elucidate the mechanism of the spheronization of pharmaceutical material crystals through extremely high shearing force using a mechanical powder processor, which produces spherical crystals without a solvent. The spheronization of theophylline, acetaminophen, clarithromycin, ascorbic acid and lactose was investigated, and the relationship between the spheronization mechanism and material characteristics was also examined. Theophylline and ascorbic acid crystals were...
23. January 2016
This paper describes the preparation and the release properties of composite materials based on Pluronic F127 and gelatin hydrogels, which could be of interest in the field of enteral nutrition or drug administration. The composites were prepared by exploiting the opposite responsivity to temperature of a 20% w/w Pluronic F127 aqueous solution (critical gelation temperature around 23 °C) and gelatin (gel–sol temperature transition around 30 °C). More