- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. July 2018
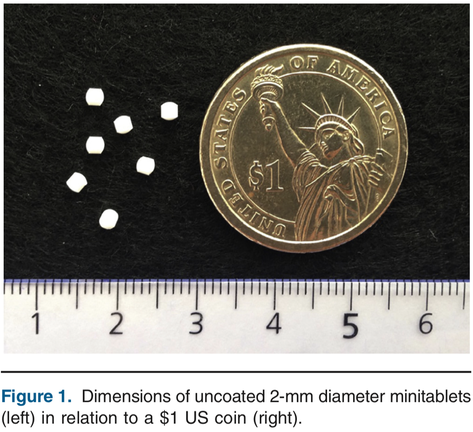
The objective of this study was to assess the acceptability and swallowability of several minitablets when administered as a unit dose compared with an equivalent dose of syrup in children aged 6 months to 5 years. Study design The acceptability and swallowability of multiple drug-free minitablets in comparison with glucose syrup was assessed in 372 children of 2 age groups (186 in age group 1 [6-23 months of age] and 186 in age group 2 [2-5 years of age]) in a randomized, 3-way, single...
28. March 2018
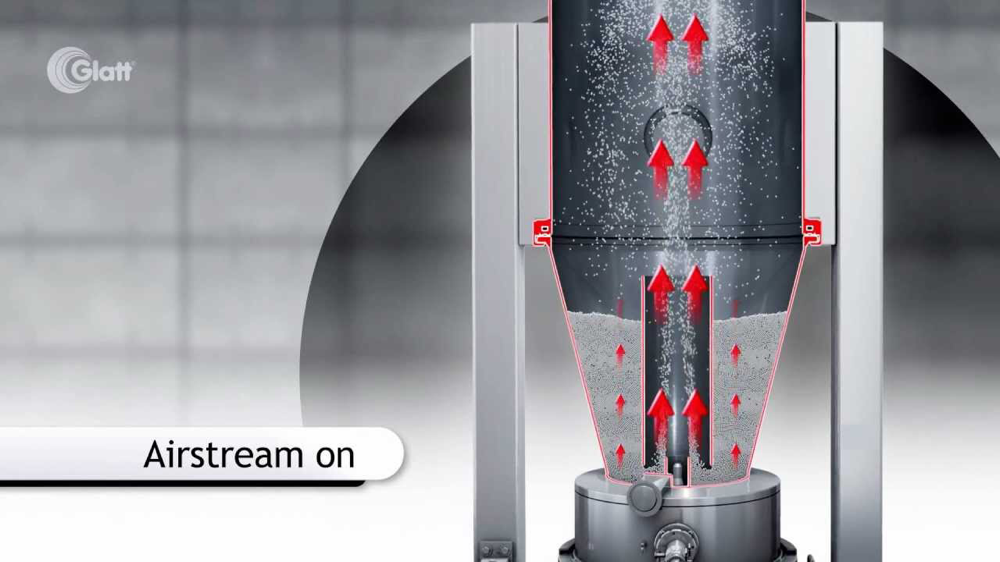
Mini-tablets with diameters of 2.0, 2.5, and 3.0 mm are coated in two different lab-scale fluidized bed coaters equipped with a Wurster draft tube. The main focus of the research is to evaluate the inter-particle coating variability, and to assess the contribution of cycle time variation. Cycle times are measured using a photoluminescent tracer with a detector mounted on the top of the draft tube. The number of passes variability is represented from 5 to 28% of the total coating variability....
02. March 2018
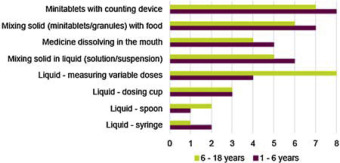
The selection and design of dosage form for the paediatric population is a real challenge. Part of that challenge is to deal with the diversity of the patient population (new born to adolescent) (CPMP, 2001) leading to specific patient compliance and acceptability. The choice of an age-appropriate formulation for children aged from 1 to 6 years old requires one to consider the ease of use of the formulation as well as the acceptability which will be mainly driven by palatability.
24. February 2018
Enalapril is an off-patent angiotensin-converting enzyme inhibitor for which no paediatric age-appropriate formulation is commercially available in Europe, and enalapril maleate (EM) orodispersible minitablets (ODMTs) have previously been formulated within the LENA (labelling enalapril from neonates to adolescents) project. In this study, a dilution method has been developed by dispersing the lowest dose strength ODMTs to enable flexible and precise EM dosing during the dose titration phase of...
04. April 2017
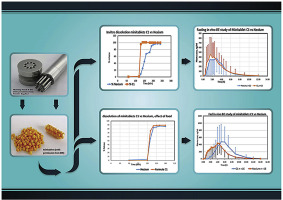
Abstract The objective of this study was to develop Esomeprazole solid dosage form as minitablets. The effect of coating thickness and percentage of fast disintegrants on the in vitro and in vivo performance of minitablets were studied. Two formulae (A1&B1) of the same core composition with different coat thickness were prepared initially. The in vivo study of A1 and B1 versus the originator revealed that their rate of dissolution was not enough to achieve bioequivalence with respect to...
19. January 2017
Abstract The oral delivery of mucoadhesive patches has been shown to enhance the absorption of large molecules such as peptides. We hypothesized that this mechanism could have utility for poorly soluble small molecules by utilizing a mucoadhesive polymer as the matrix for an amorphous solid dispersion. Binary dispersions of itraconazole and carbomer (Carbopol 71G) were prepared utilizing a thermokinetic mixing process (KinetiSol Dispersing) and the physicochemical properties were investigated...