- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
11. April 2017
Summary Topical drug application has the advantage of avoiding systemic side effects. We attempted to develop a long-acting matrix-type tablet containing indomethacin (IM) with low physical stimulus and potent mucoadhesive force to treat pain caused by oral aphtha. A mixture of polyethylene glycol (PEG) and hard fat was used as the tablet base. Ethylcellulose was added to the base in an attempt to control drug release. Tablets with PEG as a base were also prepared for comparison. Polyvinyl...
15. December 2016
Abstract Most current vaccine preparations are in injectable forms, which are inconvenient to patients and ineffective in mucosal immunization. Therefore, most research in this field has been directed at developing ideal oral vaccines enabling the induction of both systemic and mucosal immune responses. In the present study, we examined the utility of a pH-responsive polymeric carrier, poly (methacrylic acid-g-ethylene glycol) [P (MAA-g-EG)] hydrogel, as a potential oral vaccine carrier that...
22. July 2016
The use of thin film drug delivery systems is growing in importance as the benefits, such as improved drug bioavailability, reduced adverse events and avoidance of the first pass metabolism, are increasingly being recognised. Megan Greth and Scott Barnhart from ARx look in more detail at what this method can offer. Link to ONdrugDelivery Magazine
17. February 2016
A number of biobarriers limit efficient oral drug absorption; both polymer-based and lipid-based nanocarriers have demonstrated properties and delivery mechanisms to overcome these biobarriers in preclinical settings. Moreover, in order to address the multifaceted oral drug delivery challenges, polymer-lipid hybrid systems are now being designed to merge the beneficial features of both polymeric and lipid-based nanocarriers. More
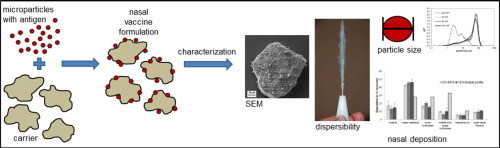
17. February 2016
Dry powder formulations for nasal vaccine delivery offer versatile advantages compared to liquid formulations, such as increased storage stability and simplified administration. The objective of the present study was the development of a dry powder nasal vaccine formulation making use of antigen-loaded chitosan microparticles. Special emphasis was put on the development and characterization of a formulation which can realistically be used in humans by means of a nasal dry powder sprayer....