- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. August 2018
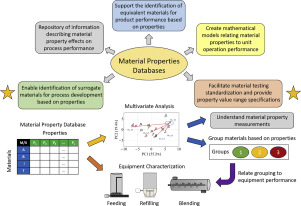
Material properties are known to have a significant impact on pharmaceutical manufacturing performance, particularly for solid product processes. Evaluating the performance of a specific material, for example an active pharmaceutical ingredient or excipient, is critical during development stages in order to determine the impact of material properties on the process. However, materials may be scarce during the early stages of process development due to high cost, unavailability, import...
17. August 2018
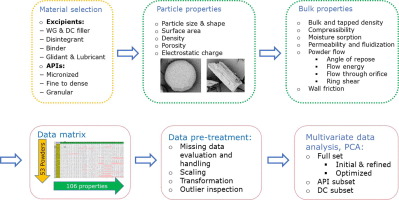
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
15. August 2018
Pharmaceutical tablets contain a variety of active substances and excipients which could be monitored by Raman spectroscopy/microscopy. Raman microscopic spectra are affected not only by the sample composition but also by preparation/measurement conditions. The character and variability of surface morphology (of tablet slices), the appropriate levels of focusing and other adjustable settings of the measurement instrumentation represent important parameters to be considered for obtaining...
16. June 2018
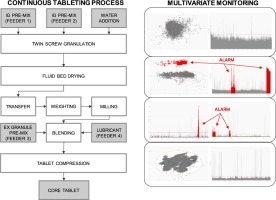
The pharmaceutical industry is undergoing a significant change in product development and manufacturing strategies with the progressive shift from batch to continuous processes. These typically feature vast volumes of data generated by the numerous sensors connected to several unit operations running over the period of several hours or even days and that demand the application of increasingly efficient tools for process understanding, monitoring and control. This paper describes the use of...
07. October 2017
Burst drug release is often considered a negative phenomenon resulting in unexpected toxicity or tissue irritation. Optimal release of a highly soluble active pharmaceutical ingredient (API) from hypromellose (HPMC) matrices is technologically impossible; therefore, a combination of polymers is required for burst effect reduction. Promising variant could be seen in combination of HPMC and insoluble Eudragits® as water dispersions. These can be applied only on API/insoluble filler mixture as...
20. March 2017
Abstract In recent years, the demand and interest for functionalized polymers have increased for drug delivery purposes. Because of the increased interest, methods that can be used to predict physical and chemical properties of polymers prior to synthesis would be of high value for the design and development of novel polymer structures. Through use of molecular descriptors and Principal Component Analysis, this study explores the possibilities of using in silico methods for polymer design and...
20. February 2017
Abstract The overall objective of this work is to understand how excipient characteristics influence the process and product performance for a continuous twin-screw wet granulation process. The knowledge gained through this study is intended to be used for a Quality by Design (QbD)-based formulation design approach and formulation optimization. A total of 9 preferred fillers and 9 preferred binders were selected for this study. The selected fillers and binders were extensively characterized...
06. June 2016
Application of thermogravimetry (TG) alone to study compatibility/incompatibility of active pharmaceutical ingredients (APIs) with excipients yields to misleading results due to overlapping of the thermal stages in the course of decomposition of both ingredients and their pharmaceutical mixtures. Hence, the purpose of this study was to assess the usefulness of multivariate statistical analysis as a supporting tool for interpretation of the TG traces during assessing compatibility of...
28. May 2016
Microscale freeze-drying makes rapid process cycles possible for early-stage formulation development. To investigate the effects of equipment scale and cooling rate on the solid state properties and the protein’s secondary structure of a sample, three binary formulations of catalase were prepared and freeze-dried with sucrose, mannitol, or (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD). The protein’s secondary structure was assessed using attenuated total reflection Fourier transform infrared...
22. March 2016
The International Conference on Harmonization Q8 (R2) includes a requirement that “Critical formulation attributes and process parameters are generally identified through an assessment of the extent to which their variation can impact the quality of the drug product,” that is, the need to assess the robustness of a formulation. In this article, a quality-by-design–based definition of a “robust formulation” for a biopharmaceutical product is proposed and illustrated with a case study....