- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
17. July 2018
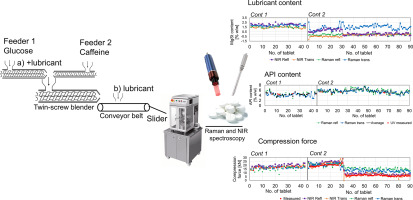
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
07. July 2018
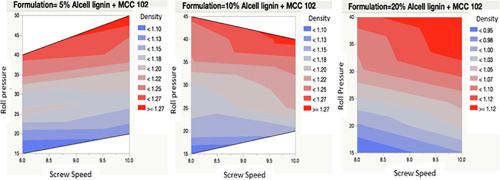
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
30. September 2017
Within the pharmaceutical industry, inline near infrared (NIR) spectroscopy is one of the most commonly applied process analytical technologies to monitor content uniformity of powder blends (1). Inline NIR provides direct insight into blending operations with respect to the degree of uniformity of the ingredient of interest either for process development or control during commercial manufacturing. A quantitative calibration for a specific ingredient may be applied to predict the content of...
20. February 2017
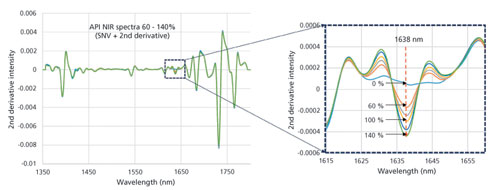
Abstract Near-infrared chemical imaging (NIR-CI) with high-speed cameras based on the push-broom acquisition principle is a rapidly-evolving and can be used for a variety of purposes, from classification (and sorting) of products to mapping spatial distribution of materials. The present study examined if NIR-CI is suitable for tablet manufacturing. To that end, the tablets were introduced into the CI system via a flat belt conveyor. A formulation, which consisted of 4wt.%–6wt.% caffeine,...
09. January 2016
he aim of the study was to investigate the effect of novel polymer/lipid formulations on the dissolution rates of the water insoluble indomethacin (INM), co-processed by hot melt extrusion (HME). Formulations consisted of the hydrophilic hydroxypropyl methyl cellulose polymer (HPMCAS) and stearoyl macrogol-32 glycerides—Gelucire 50/13 (GLC) were processed with a twin screw extruder to produce solid dispersions. More
03. June 2015
Hua Ma, Senior Development Scientist, GSK Yu-E Zhang, Senior Manager, Ferring Pharmaceuticals Hung Tian, Technical Development Leader, Novartis Hitesh Chokshi, Ph.D., Senior Research Director, Hoffmann-La Roche