- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
24. August 2018
A process control system based on PAT can compensate for variations in particle size, resulting in more consistent coating thickness.
Drug-layered multiparticulates are a common dosage form for extended or modified-release pharmaceutical formulations. Delivered either in capsules, tablets, or as food additives in pediatric or geriatric applications (1), these formulations typically feature a functional coating designed to delay dissolution of the drug in the body.
10. August 2018
Sticking and picking during tablet manufacture has received increasing interest recently, as it causes tablet defects, downtime in manufacturing, and yield losses. The capricious nature of the problem means that it can appear at any stage of the development cycle, even when it has been deemed as low risk by models, tests, and previous experience. In many cases, the problem manifests when transferring the process from one manufacturing site to another. Site transfers are more common now than in...
08. August 2018
Three-dimensional printing (3DP) has the potential to cause a paradigm shift in the manufacture of pharmaceuticals, enabling personalised medicines to be produced on-demand. To facilitate integration into healthcare, non-destructive characterisation techniques are required to ensure final product quality. Here, the use of process analytical technologies (PAT), including near infrared spectroscopy(NIR) and Raman confocal microscopy, were evaluated on paracetamol-loaded 3D printed cylindrical...
07. June 2018
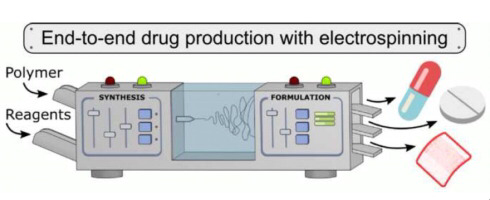
Based on the concept of continuous manufacturing an end-to-end benchtop device was developed unprecedented for the production of solid drug dosage forms by connecting flow synthesis and formulation via electrospinning (ES). Together with the optimized two-step continuous-flow synthesis of acetylsalicylic acid (ASA) a water-soluble polymeric excipient (polyvinylpyrrolidone K30, PVPK30) was introduced. The resulting polymeric solution could be readily electrospun into solid nanofibers with high...
05. March 2018
This study presents a framework for process and product development on a continuous direct compression manufacturing platform. A challenging sustained release formulation with high content of a poorly flowing low density drug was selected. Two HPMC grades were evaluated as matrix former: standard Methocel CR and directly compressible Methocel DC2. The feeding behavior of each formulation component was investigated by deriving feed factor profiles.
01. March 2018
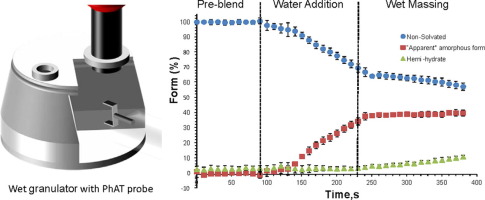
Form changes during drug product processing can be a risk to the final product quality in terms of chemical stability and bioavailability. In this study, online Raman spectroscopy was used to monitor the form changes in real time during high shear wet granulation of Compound A, a highly soluble drug present at a high drug load in an extended release formulation. The effect of water content, temperature, wet massing time and drying technique on the degree of drug transformation were examined.
21. November 2017
Process Analytical Technology, or “PAT” is the term given to analytical instruments developed to measure certain attributes of product within the manufacturing process, eliminating, or substantially minimising the need for sampling for off-line analysis. This approach has several key advantages over traditional off-line analysis methods and includes process measurements in situ with instant access to data which facilitates rapid decisions during product development and manufacture.
20. November 2017
The shift toward continuous manufacturing among European pharmaceutical manufacturers has not been accompanied by a similar strong increase in the use of automation and sensor-based on-line monitoring.
18. November 2017
by Innopharmatechnology & Glatt @ PMEC India 2017 Learn about the latest Process Analytical Technology (PAT) technical and application developments for in-line PSD and Moisture measurement in fluid bed batch and continuous powder processes. More
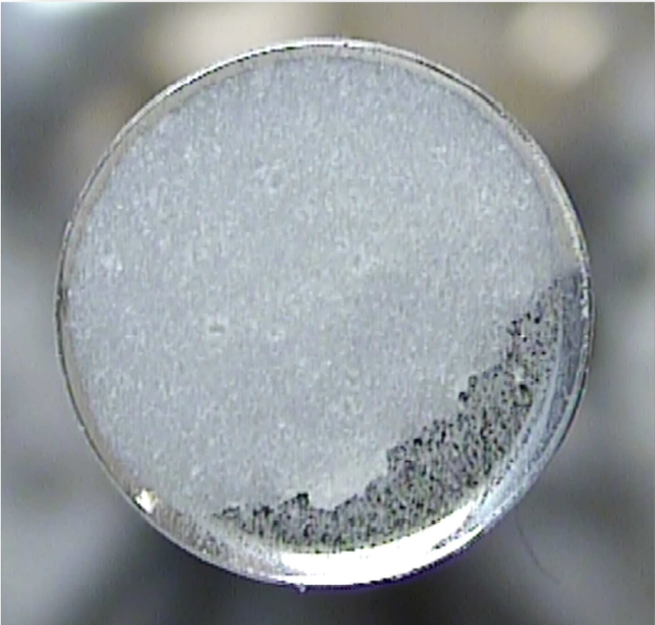
15. October 2017
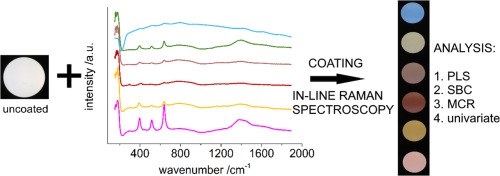
Endpoints of coating processes for colored tablets were determined using in-line Raman spectroscopy. Coatings were performed with six commercially available formulations of pink, yellow, red, beige, green and blue color. The coatings were comprising pigments and/or dyes, some causing fluorescence and interfering the Raman signal. Using non-contact optics, a Raman probe was used as process analytical technology (PAT) tool, and acquired spectra were correlated to the sprayed mass of aqueous...