- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
24. February 2018
Enalapril is an off-patent angiotensin-converting enzyme inhibitor for which no paediatric age-appropriate formulation is commercially available in Europe, and enalapril maleate (EM) orodispersible minitablets (ODMTs) have previously been formulated within the LENA (labelling enalapril from neonates to adolescents) project. In this study, a dilution method has been developed by dispersing the lowest dose strength ODMTs to enable flexible and precise EM dosing during the dose titration phase of...
22. February 2018
A small amount of food is commonly used to aid administration of medicines to children to improve palatability and/or swallowability. However the impact of this co-administered food on the absorption and subsequent pharmacokinetic profile of the drug is unknown. Existing information on food effects is limited to standard protocols used to evaluate the impact of a high fat meal in an adult population using the adult medication. In the absence of a substantial body of data, there are no specific...
18. November 2017
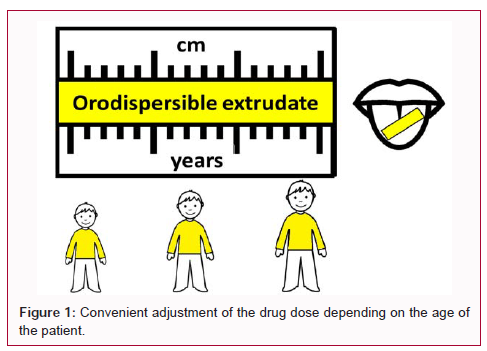
The aim of the present investigation is to produce rapidly disintegrating laminar extrudates for delivering ibuprofen in the mouth of paediatric patients. The laminar shape is particularly convenient for drug delivering in the mouth and can be easily cut in different sizes allowing for a convenient adjustment of the drug dose depending on the age of the patient. Due to the fact that in paediatric formulations, the selection of the excipients is always a challenging issue and the reduction of...
08. March 2017
Introduction Inhaled mannitol has beneficial effects on lung function, mucociliary clearance, quality of life and sputum properties. This trial examined the efficacy of inhaled mannitol in children with cystic fibrosis (CF). Methods The efficacy of inhaled mannitol in children with CF aged 6–17 years was assessed in a phase 2, randomised, placebo-controlled crossover study. Subjects were randomly assigned to mannitol 400 mg every 12 h or matching placebo for 8 weeks, followed by an 8 week...
24. January 2017
ABSTRACT The lack of age-appropriate (paediatric) authorised medicines is a long-standing problem amongst regulatory authorities, patients, parents and prescribers. This is driven by the paucity of information on clinical efficacy, deficiency in safety data (i.e. biopharmaceutics) and the lack of quality information such as palatability and acceptability data in children. To counteract this deficiency bespoke, unlicensed formulations are formulated by contract manufacturers, hospitals and...
10. October 2016
Abstract A lack of evidence to guide the design of age-appropriate and acceptable dosage forms has been a longstanding knowledge gap in paediatric formulation development. The Children’s Acceptability of Oral Formulations (CALF) study captured end-user perceptions and practices with a focus on solid oral dosage forms, namely tablets, capsules, chewables, orodispersibles, multiparticulates (administered with food) and mini-tablets (administered directly into the mouth). A rigorous development...
10. August 2016
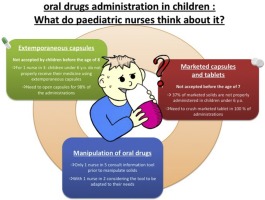
Abstract The purpose of this study was to interview paediatric nurses on administration issues using extemporaneous capsules and marketed capsules and tablets in children younger than 6 years old, based on most frequently administered drugs in six participating wards. The 59 responding nurses estimated respectively at 7.7 ± 1.7 and 7.3 ± 1.8 years the age from which children would properly swallow extemporaneous capsules and marketed solids, with 33% and 37% of nurses considering that...
09. August 2016
Abstract This study involves the development of thin oral solvent cast films for the potential delivery of the proton pump inhibitor, omeprazole (OME) via the buccal mucosa for paediatric patients. OME containing films were prepared from ethanolic gels (1% w/w) of metolose (MET) with polyethylene glycol (PEG 400) (0.5% w/w) as plasticiser, and l-arg (0.2% w/w) as a stabilizer and dried in an oven at 40 °C. The blank and drug loaded films were divided into two groups, one group was subjected to...
09. August 2016
The importance of providing safe and effective delayed- and extended-release oral formulations that can replace products requiring multiple administrations has been continually cited as an area in need of improvement for pharmaceutical companies. Such controlled release challenges become especially critical when they must be adapted for paediatrics, those suffering from dysphagia, or patients with specific dosage administration limitations. More often than not, lack of palatability and...
03. August 2016
Objectives Conduct a preliminary comparison of the bioavailability between two formulations: commercial grade coenzyme Q10 (CoQ10) powder (solid formulation) and a new oil-in-water liquid emulsion and their effect on other antioxidants. Methods Six healthy individuals participated in a randomized, crossover, open, consecutive design, with a 2-week washout period. Pharmacokinetic parameters were assessed after a single and multiple intakes of 250 mg CoQ10 given daily for 1 week. Key Findings The...