- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
08. August 2018
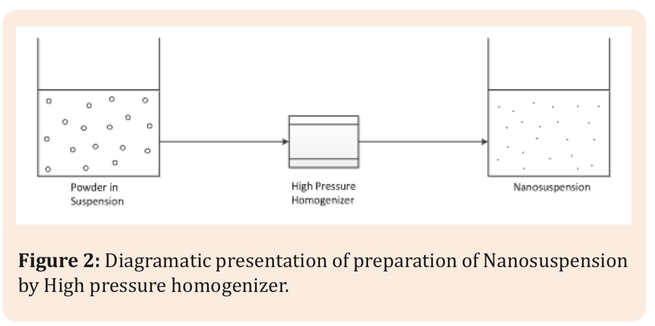
Combinatorial chemistry, computational molecular modeling and high throughput screening in drug discovery have significantly increased the number of poorly soluble drugs. About 40% of drugs developed in the past and about 90% of the drugs in development are poorly soluble drugs. When administered orally, a drug has to first dissolve in gastrointestinal fluids before it can be absorbed in to the blood and reach its site of action. The objective of this review article is to outline the key...
02. May 2018
An increase in the proportion of poorly aqueous soluble drugs that exhibit problems in oral bioavailability is one of the major problems in formulation development. Formulation of lipid-based nanocarriers, such as self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) have received a lot of attention in recent years as an approach for overcoming poor solubility and oral bioavailability of drugs. SNEDDS are isotropic mixtures of oil,...
14. November 2017
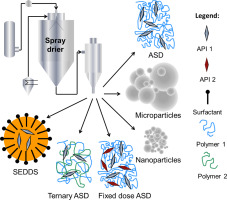
Poorly water-soluble drugs are a significant and ongoing issue for the pharmaceutical industry. An overview of recent developments for the preparation of spray-dried delivery systems is presented. Examples include amorphous solid dispersions, spray dried dispersions, microparticles, nanoparticles, surfactant systems and self-emulsifying drug delivery systems.
07. August 2017
A suitable dissolution test as a surrogate for in vivo absorption is highly attractive in the early stage of formulation development. Ideally, changes in dissolution in vivo should be reflected by the corresponding in vitro release. However, conventional dissolution tests have limitations to address this need due to the lack of biorelevance.