- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
02. March 2017
Abstract A new and highly biocompatible space holder material is proposed for manufacturing of porous titanium with open and interconnected pore morphologies through powder metallurgy techniques. Sugar pellets are compacted with titanium powder and then removed by dissolution in water before sintering. The morphology, pore structure and porosity were observed by optical microscopy, SEM and micro-CT. The porous titanium has highly spherical pore shapes, well-controlled pore sizes and high...
22. June 2016
Abstract Malaria is a parasitic and vector determined blood-conceived infectious disease transmitted through infected mosquitoes. Anti-malarial drug resistance is a major health problem, which hinders the control of malaria. Drug-resistant malaria when surveyed, the results demonstrated safe proclivity to nearby all anti-malarial regimes accessible except from artemisinin and its derivatives. Artemether is a BCS class IV drug effective against acute and severe falciparum malaria hence; there is...
21. June 2016
Abstract Mucoadhesive microparticles formulated in a capsule and delivered to the gastrointestinal tract might be useful for local drug delivery. However, swelling and agglomeration of hydrophilic polymers in the gastrointestinal milieu can have a negative influence on particle retention of mucoadhesive microparticles. In this work, we investigated the impact of dry-coating with nano-sized hydrophilic fumed silica on dispersibility and particle retention of mucoadhesive microparticles. As a...
09. April 2016
The aim of this study was to prepare and evaluate solid dispersion tablets containing a poorly water-soluble drug using porous calcium silicate (PCS) by a wet granulation method. Nifedipine (NIF) was used as the model poorly water-soluble drug. Solid dispersion tablets were prepared with the wet granulation method using ethanol and water by a high-speed mixer granulator. The binder and disintegrant were selected from 7 and 4 candidates, respectively. The dissolution test was conducted using the...
13. March 2016
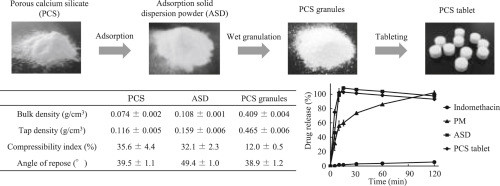
Solid dispersion techniques are useful for improving the dissolution of poorly water-soluble drugs. This study aimed to produce and evaluate solid dispersion tablets improving the solubility and oral bioavailability of a poorly water-soluble indomethacin (IND) by wet granulation method with a high-speed mixer granulator combined with porous calcium silicate (PCS). A low density of PCS is a major disadvantage which is a bulky volume and scattering. So, it is necessary to prepare a high density...
27. February 2016
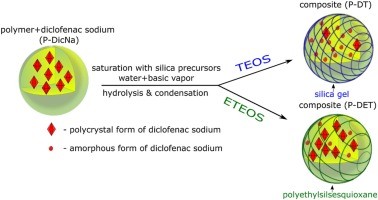
The present study concerns the preparation of ternary composites via the in situ encapsulation of solid dispersion of diclofenac sodium within the acrylic polymer beads. The encapsulating species were produced through the hydrolysis and condensation of the silica precursors (tetraethoxysilane or ethyltriethoxysilane) introduced into the solid dispersion. The transformation of precursors occurred in the vapor phase of ammonia. A great advantage of the presented vapor-phase method is preventing...
15. January 2016
A new formulation method for solid dosage forms with drug loadings from 0.65 ± 0.03% to 39 ± 1% (w/w) of acetaminophen (APAP) as a model drug has been presented. The proposed method involves the production of highly-porous lactose with a BET surface area of 20 ± 1 m2/g as an excipient using a templating method and the incorporation of drug into the porous structure by adsorption from a solution of the drug in ethanol. More
04. October 2015
Calcium carbonate can be 'functionalized' by use of etching agents such as phosphoric acid to create inter- and intra-particle porosity with a range of morphologies. Functionalized calcium carbonate has potential for use as a carrier for the delayed release of actives, such as drugs, plant protection chemicals, and food additives such as flavors. The drug or flavor is released slowly by permeation and diffusion. More
23. June 2015
A novel tablet product that in an easy, flexible and reproducible manner can be loaded with a relatively high amount of a pharmaceutically acceptable liquid formulation e.g. carrying a therapeutically, prophylactically and/or diagnostically active substance. The novel loadable tablet product may be produced in large-scale batches and stored until use and each batch or sub-batch may be loaded with the same or different pharmaceutically acceptable liquid formulations and/or active substances. A...