- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
22. September 2016
Abstract: Background: In this era of evolution, quality and safety of pharmaceutical dosage forms have been given a prime importance. In this context, a detailed knowledge about physical and chemical properties of excipients is not sufficient, but information about safety and regulatory status of these materials is now essential to know. The present work will be beneficial to focus more on the safety, quality and stability of pharmaceutical product. Objective: Study of regulatory mechanism for...
10. August 2016
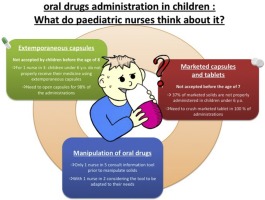
Abstract The purpose of this study was to interview paediatric nurses on administration issues using extemporaneous capsules and marketed capsules and tablets in children younger than 6 years old, based on most frequently administered drugs in six participating wards. The 59 responding nurses estimated respectively at 7.7 ± 1.7 and 7.3 ± 1.8 years the age from which children would properly swallow extemporaneous capsules and marketed solids, with 33% and 37% of nurses considering that...
27. June 2016
Abstract Pharmaceutical excipients contribute unique functionalities to formulations, thereby largely determining the drug products quality and influencing its safety and efficacy. Changes and variations of excipients in licensed products are, therefore, placed under strict regulatory control. This article presents a proposed regulatory mechanism for pharmaceutical excipients by United States of Food and Drug Administration (USFDA). In Unites States, excipients are regulated by United States of...
28. January 2016
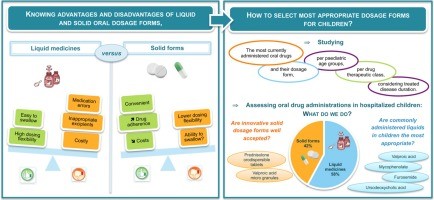
Selecting the most appropriate dosage form, that ensures safe administration and adherence of medications, is a major issue for children. Marketed drugs, however, have rarely been tested for their use in children. There is a need for more data on drug formulations administered to children to identify unmet needs, and drive future paediatric research. More