- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
08. August 2018
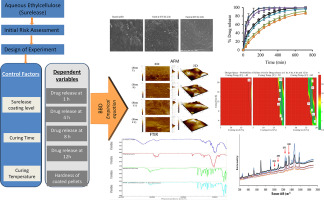
The main purpose of this study was to investigate the influence of ethylcellulose (EC) coating and curing conditions on the quality attributes of pharmaceutical pellets prepared using a quality by design (QbD) approach. The drug release rate of methimazole, a freely soluble model drug, was evaluated extensively together with the mechanical strength and true density of the coated pellets. Moreover, the thermal and spectroscopic properties, as well as the surface characteristics were studied...
07. July 2018
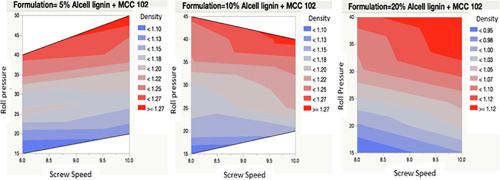
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
02. July 2018
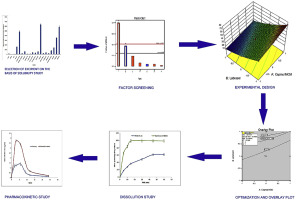
The aim of this study was to develop self-nanoemulsifying drug delivery system (SNEDDS) of bosentan using quality by design (QBD) approach with better bioavailability. The major component of the formulation vis-à-vis lipid (Capmul MCM), surfactant (LABRASOL) and co-surfactant (PEG 600) were selected on the basis of saturation solubility. Mixture of LABRASOL and PEG 600 in the ratio of 1:1 showed better nano emulsifying region as depicted by pseudo ternary phase diagram. The optimum mixture of...
26. May 2018
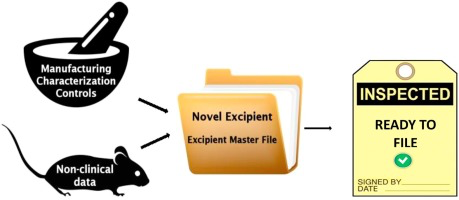
Novel excipients are indispensable in development of modern, advanced drug delivery systems and biotechnology-derived drugs. Although numerous novel excipients are developed for pharmaceutical use, they are not frequently seen in medicinal products due to the strict regulatory requirements and perception that their use makes new product evaluation more complex with risk of delays in the approval process. Regulators regard novel excipients as new substances and whenever new excipient is used in...
17. May 2018
Current in vitro disintegration methods for polymeric films are qualitative and introduce significant user bias. The goal of these studies is to develop a novel, quantitative disintegration technique which can be used to characterize polymeric films in vitro. Methods A method was developed using a Texture Analyzer instrument to evaluate film disintegration. Solvent-casted, clinically advanced, anti-HIV, vaginal films as well as marketed vaginal films were used throughout these studies. Method...
15. December 2017
Formulators usually face three kinds of challenges while addressing sensory quality. Firstly, when existing products are changed, the sensory properties should feel the same for customers and patients. In addition to that, sensory problems like formulations that are too greasy, sticky or contain other negative feelings must be tackled. Lastly, you should send positive and beneficial sensory messages to the patients while designing the formulation.
28. November 2017
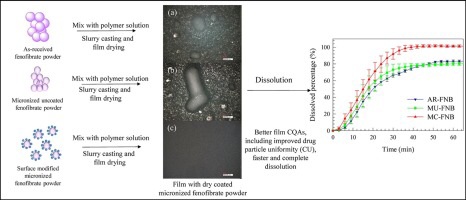
Recent work has established polymer strip films as a robust platform for delivery of poorly water-soluble drugs via slurry casting, in particular using stable drug nanosuspensions. Here, a simpler, robust method to directly incorporate dry micronized poorly water-soluble drug, fenofibrate (FNB), is introduced. As a major novelty, simultaneous surface modification using hydrophilic silica along with micronization was done using fluid energy mill (FEM) in order to reduce FNB hydrophobicity and...
13. November 2017
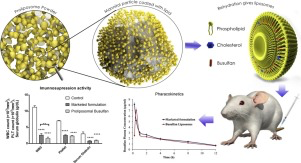
Parenteral administration of Busulfan (BU) conquers the bioavailability and biovariability related issues of oral BU by maintaining the plasma drug concentration in therapeutic range with minimal fluctuations thereby significantly reducing the side effects. Busulfex® is the only commercially available parenteral formulation of BU composed of organic solvents N, N-dimethylacetamide and polyethylene glycol 400. Since, BU is highly susceptible to hydrolytic degradation; Busulfex® has poor...
27. September 2017
Because of the multiple roles they play in drug product formulations, excipient quality is as important as that of the API, so standards are needed to ensure their purity and reliability.
26. September 2017
Examining the implications and practical implementation of multi-disciplinary International Conference on Harmonization (ICH) topics, this book gives an integrated view of how the guidelines inform drug development strategic planning and decision-making.