- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
08. November 2017
Colon delivery systems for oral administration have grown in popularity since the 1990s, primarily because of the increasing incidence of inflammatory bowel disease (IBD) that has broadly been demonstrated to benefit from topical pharma- cological treatment.
06. November 2017
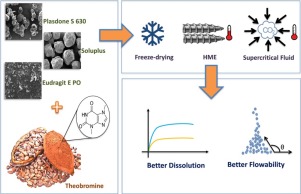
The aim of this study was to improve the pharmaceutical properties of theobromine (TB), particularly its flowability and dissolution rate, by preparing solid dispersions using different technologies (hot melt extrusion—HME, freeze-drying—FD, and supercritical fluid—SF) as well as testing different hydrophilic polymeric matrixes (Eudragit™ E, Plasdone™ S and Soluplus™)
05. April 2017
Abstract We investigated the effectiveness of using Carr’s flowability index (FI) and practical angle of internal friction (Φ) as indexes for setting the target Mg-St mixing time needed for preparing tablets with the target physical properties. We used FI as a measure of flowability under non-loaded conditions, and Φ as a measure of flowability under loaded conditions for pharmaceutical powders undergoing direct compression with varying concentrations of Mg-St and mixing times. We evaluated...
18. September 2016
Inflammatory bowel disease (IBD) is an idiopathic, relapsing disease involving chronic inflammation of the digestive tract, either in part or entire. IBD primarily includes Crohn’s disease (CD) and ulcerative colitis (UC) with a high prevalence rate in the industrialized world, with North America noting the highest frequency of people suffering with CD [1]. Incidence of IBD is from 31 to 71 per100,000 people for CD and 18-31 per 100,000 for UC and is increasing at an alarming rate. Although,...
08. August 2016
The present investigation was carried out to develop and characterize a multifunctional co-processed excipient for improving the compressibility of poorly compressible drugs. Etodolac was used as a model drug. Microcrystalline cellulose (MCC), lactose monohydrate (lactose), and StarCap 1500 (StarCap) were selected as components of the co-processed excipient. The spray drying method was used for co-processing of excipients. D-optimal mixture design was applied to optimize the proportion of...
09. May 2016
An ultrasound measurement system was employed as a non-destructive method to evaluate its reliability in predicting the tensile strength of tablets and investigate the ben- efits of incorporating it in a continuous line, manufacturing solid dosage forms. Tablets containing lactose, acetaminophen, and magnesium stearate were manufactured contin- uously and in batches. The effect of two processing parameters, compaction force and level of shear strain were examined. Young’s modulus and tensile...
08. May 2016
The aim of the current study was to formulate and evaluate glipizide controlled release matrix tablets by means of different grades of polymer Ethoceland different co-excipients in order to evaluate their effect on drug release profiles during in vitro dissolution studies. Type II diabetes mellitus is usually treated with Glipizide. Glipizide belongs to sulfonylurea group. Gastric disturbance and severe hypoglycemia has been observed after taking glipizide orally. To overcome these problems,...
10. March 2016
ABSTRACT Six batches of formulated sustained release matrix tablets of Cefixime Trihydrate were prepared by using different polymers like Tamarind Gum, Carnauba wax, HPMC, MCC, PVP K30 by wet granulation technique. The effects of prepared tablets by using natural polymers were evaluated for pre compression parameters, uniformity of content, assay and in vitro drug release. Influences of different parameters like pH, agitation intensity on drug release were also studied. Cefixime Trihydrate...
19. January 2016
The thiolation of polyallylamine (PAAm) for use in mucoadhesive drug delivery has been achieved. PAAm was reacted with different ratios of Traut's reagent, yielding products with thiol contents ranging from 134–487 μmol/g. Full mucoadhesive characterisation of the thiolated PAAm samples was conducted using swelling studies, mucoadhesive testing on porcine intestinal tissue and rheology. Both swelling and cohesive properties of the thiolated PAAm products were vastly improved in comparison to...
23. December 2015