- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
03. September 2018
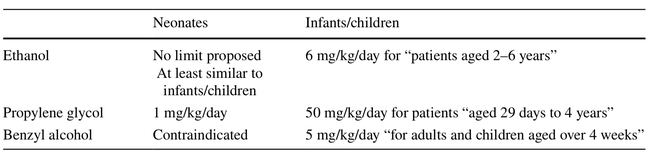
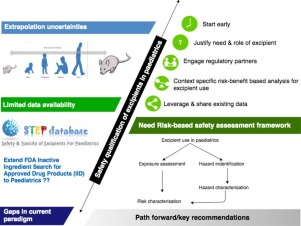
To attain effective and safe pharmacotherapy, formulations in (pre)term neonates should enable extensive dose flexibility. During product development and subsequent authorization and clinical use of such formulations, there is also a need for informed decisions on excipient exposure: in addition to the need to improve the knowledge on active compounds, there is a similar need to improve the knowledge on excipients in neonates. Excipients are added to formulations as co-solvent, surfactant,...
16. July 2018
Dear readers, It’s hard to believe that we’re issuing our June publication already and we find ourselves heading for the summer break. And of course, we’re now preparing for those mid-year reviews to measure progress to ensure we can meet our goals by the end of the year. Not only that but September is not far away when the Board will convene with an eye to 2019. We’ll be revisiting our Agenda 2020 to confirm our strategic direction and that we’re working on the right things to get...
16. November 2017
A public workshop entitled "Challenges and strategies to facilitate formulation development of pediatric drug products" focused on current status and gaps as well as recommendations for risk-based strategies to support the development of pediatric age-appropriate drug products. Representatives from industry, academia, and regulatory agencies discussed the issues within plenary, panel, and case-study breakout sessions.
21. July 2017
A public workshop entitled “Challenges and strategies to facilitate formulation development of pediatric drug products” focused on current status and gaps as well as recommendations for risk-based strategies to support the development of pediatric age-appropriate drug products.