- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. June 2018
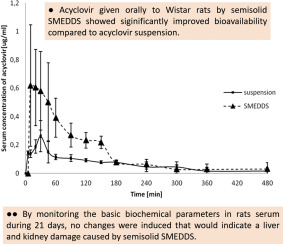
Semisolid self-microemulsifying drug delivery system (SMEDDS) with optimized drugloading capacity, stability, dispersibility in aqueous media and invitro drug release profile, was evaluated in vivo regarding effects on pharmacokinetics of acyclovir, an antiviral with low bioavailability (BA) and short half-life (t1/2). Additional goal of this study was evaluation of safety of this semisolid SMEDDS consisted of medium chain length triglycerides (oil) (10% w/w), macrogolglycerol hydroxystearate...
18. May 2018
The European Food Safety Authority (EFSA) will advise the Commission on whether to re-evaluate titanium dioxide by analysing four scientific studies that questioned the additive's safety. Article from Foodnavigator.com
16. November 2017
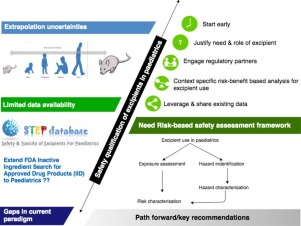
A public workshop entitled "Challenges and strategies to facilitate formulation development of pediatric drug products" focused on current status and gaps as well as recommendations for risk-based strategies to support the development of pediatric age-appropriate drug products. Representatives from industry, academia, and regulatory agencies discussed the issues within plenary, panel, and case-study breakout sessions.
29. September 2017
Ibuprofen is a non-steroidal anti-inflam-matory drug frequently administered to children of var-ious ages for relief of fever and pain and is approved as over-the-counter medication in many countries world-wide. Although there is extensive data on its efficacy and safety in children and adults, there are divergent dosing recommendations for analgesia and treatment of fever in infants, especially in the age group between 3 and 6 months of age.
27. September 2017
A chart-review of 200 neonates randomly selected from the Neonatal Intensive Care Unit (NICU) of the university hospital in Copenhagen revealed that ap-proximately 10% received intravenous (i.v.) paracetamol≥4 days (unpublished data). Paracetamol (acetaminophen) is commonly used to control mild-to-moderate pain or to reduce opioid exposure either by oral, rectal or intrave-nous route.
06. June 2017
This phase 3, laboratory classroom study assessed the efficacy and safety of methylphenidate hydrochloride extended-release chewable tablets (MPH ERCT) compared with placebo in children with attention-deficit/hyperactivity disorder (ADHD).
17. March 2017
Outline • Excipient safety review in generic drug applications • Bridging justifications for excipients in generic drugs – What is their utility? – What are important factors to consider? • Case Studies – Excipient grade, dose, duration, route of administration, patient population
08. March 2017
Abstract This study was designed to develop a once-daily controlled-release matrix tablet of aceclofenac 200 mg (AFC-CR) with dual release characteristics and to investigate the role of an alkalizer in enhancing drug solubility and reducing the occurrence of gastroduodenal mucosal lesions. Two formulation approaches were employed, namely a monolithic matrix tablet and a bilayered tablet. In vitro dissolution studies of AFC-CR tablets were carried out in simulated intestinal fluid (pH 6.8...
30. September 2016
Abstract Combining two or more antiretroviral drugs in one medical product is an interesting but challenging strategy for developing topical anti-HIV microbicides. We developed a new vaginal delivery system comprising the incorporation of nanoparticles (NPs) into a polymeric film base – NPs-in-film – and tested its ability to deliver tenofovir (TFV) and efavirenz (EFV). EFV-loaded poly(lactic-co-glycolic acid) NPs were incorporated alongside free TFV into fast dissolving films during film...
04. June 2016
Objective To evaluate the safety and explore the efficacy of recombinant human lactoferrin (talactoferrin [TLf]) to reduce infection. Study design We conducted a randomized, double blind, placebo-controlled trial in infants with birth weight of 750-1500 g. Infants received enteral TLf (n = 60) or placebo (n = 60) on days 1 through 28 of life; the TLf dose was 150 mg/kg every 12 hours. Primary outcomes were bacteremia, pneumonia, urinary tract infection, meningitis, and necrotizing enterocolitis...