- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
01. September 2018
Flow properties of microcrystalline cellulose (MCC) excipients, Avicel PH 101 and Avicel PH 102 have been compared by using Brookfield PFT. Afterwards, the effect of hydrophobic Silica R972 as glidant has been tested on both the excipients. Hand blending is done by mixing MCCs with hydrophobic silica R972 vigorously by SAC as an underlined basis for 10 minutes and the flow properties tests are performed. During the flow function test “as received” Avicel PH 102 shows better flow function...
20. April 2018
Silica-based nanoparticles are used as excipients in pharmaceutical technology. Recently, mesoporous silica nanoparticles have emerged as drug delivery systems. Their porous structure enables the high drug-loading of drugs with poor water solubility. The silica matrix protects entrapped drugs against enzymatic degradation. Furthermore, the premature release of drugs is hindered by pore-gating strategies. Adding a targeting ligand to the silica-based nanoparticles directs them to diseased cells...
05. March 2018
This study presents a framework for process and product development on a continuous direct compression manufacturing platform. A challenging sustained release formulation with high content of a poorly flowing low density drug was selected. Two HPMC grades were evaluated as matrix former: standard Methocel CR and directly compressible Methocel DC2. The feeding behavior of each formulation component was investigated by deriving feed factor profiles.
23. February 2018
The study concerns the synthesis and application of a novel type of ZrO2-SiO2 binary oxide system. The material was prepared via a sol-gel route, using zirconium(IV) propoxide and tetraethyl orthosilicate solutions as zirconia and silica precursors, ammonia as a promoter of hydrolysis, and ethanol as a solvent. Different amounts of reactants were used to obtain samples with ZrO2:SiO2 molar ratios of 1:1, 4:1 and 1:4. The synthesized oxide materials were additionally calcined at 1000 ºC to...
26. December 2017
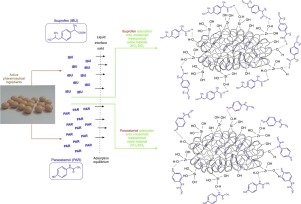
This study presents an interesting and promising strategy for producing an oral multiparticulate formulation of the sustained-release of diclofenac sodium (DS) consisting of subunits closed inside hard gelatin capsules (each capsule contains ~ 50 mg of diclofenac sodium). The subunits in the form of beads were produced through the encapsulation of diclofenac sodium dispersed within a nondisintegrating polymer carrier by a silica gel functionalized with the 3-aminopropyl groups.
22. December 2017
Grace’s SILSOL® technology offers solutions for poorly soluble active ingredients. Based on compendial silica and fully scalable, it gives pharmaceutical developers new options to enhance bioavailability of BCS2 compounds, especially during early research and discovery.
03. December 2017
Excipients with good flowability, bulk density as well as compaction properties are desired for use in tableting since they play important roles in formulation development and processing, including, handling, mixing, feeding and compaction.
30. October 2017
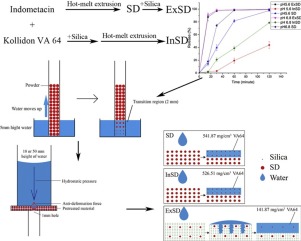
The purpose of this work was to investigate the effect on the dissolution behavior when silica was added in different ways. The solid dispersion was prepared by hot-melt extrusion (HME) using indomethacin (IND) as a model drug and Kollidon VA64 as a carrier.
18. October 2017
WR Grace & Co has licensed rights to a bioavailability enhancement technology from Formac Pharmaceuticals NV.
11. October 2017
TruTag Technologies guides pharmaceutical and nutraceuticalcompanies in navigating the complexities of the supply chain by mitigating risks due to unauthorized diversion, counterfeiting, product recalls and quality incidents. More