- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
02. October 2018
The formulation of amorphous solid dispersions (ASDs) is an effective way to improve the bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs). The combination of an amorphous state of the drug and the presence of crystallization-inhibiting polymers retains a high amount of dissolved API over time. ASDs with ketoconazole and different polymers were manufactured by spray drying and their characteristics as well as performance were analyzed. Dissolution tests with a...
12. September 2018
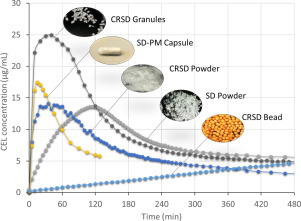
The amorphous solid dispersion (ASD) technique has been employed to formulate poorly-soluble drugs, however, development of solid dosage forms with ASD is challenging due to the high propensity of amorphous drug to precipitate upon dissolution. Thus this work aimed to explore the potential of controlled release amorphous solid dispersion (CRASD) systems using polyvinyl acetate (PVAc) as a release-retarding excipient to mitigate the drug precipitation during dissolution of poorly water-soluble...
07. September 2018
Lipid based formulations are known to enhance the solubility of drug compounds. The purpose of this study was to determine the intrinsic solubility enhancement of several drug compounds in the presence of a lipid based formulation at 37°C. The ratio of the components in the formulation were varied to investigate the effect of composition on the solubility of the compound. Conclusion Using CheqSol, it was possible to readily evaluate the solubility of ionizable compounds at elevated...
24. August 2018
In today's drug development world, combinatorial chemistry, high-throughputscreening, and genomics have provided a technologic platform that produces a large number of new chemical entities withtherapeutic potential each year. Its outcome the new chemical entities shifted towards higher molecular weight and increasing lipophilicity that results in poor water solubility which primarily affectsthe bioavailability of orally administered drugs. Hence, the poor aqueoussolubility not only limits the...
04. August 2018
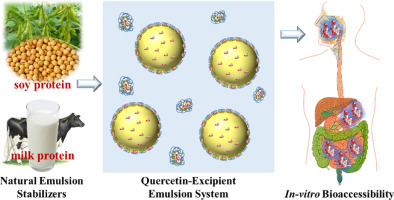
Emulsion-based excipient foods were developed to improve the bioaccessibility of an important hydrophobic nutraceutical: quercetin. Protein-stabilized oil-in-water excipient emulsions were prepared using sodium caseinate, whey protein isolate, or soy protein isolate as an emulsifier. These emulsions were then mixed with powdered quercetin and heated to simulate a cooking process. The excipient emulsions had relatively small droplet sizes (d < 270 nm) and remained stable against...
31. July 2018
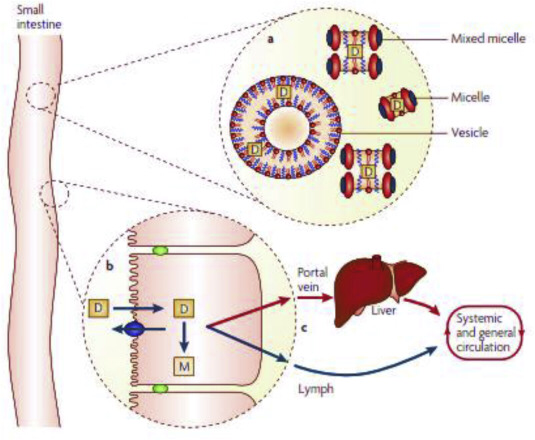
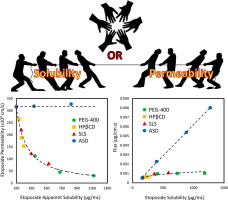
Poor aqueous solubility is a major challenge in today's biopharmaceutics. While solubility-enabling formulations can significantly increase the apparent solubility of the drug, the concomitant effect on the drug's apparent permeability has been largely overlooked. The mathematical equation to describe the membrane permeability of a drug comprises the membrane/aqueous partition coefficient, which in turn is dependent on the drug's apparent solubility in the GI milieu, suggesting that the...
30. July 2018
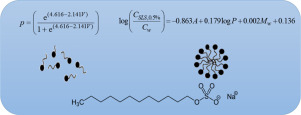
Micellar solubilization is a great method for increasing drugs solubility in aqueous environments. At concentrations above the critical micelle concentration (CMC), micelles are formed and they are able to increase the apparent aqueous solubility of poorly soluble drugs. Sodium lauryl sulfate (SLS) is one of the common solubilizing agents in pharmaceutical sciences. Investigation on the water solubility of drugs in the presence of surfactants and the development of a relationship between drug...
20. July 2018
Soluplus® has been approved in generics in Taiwan and Argentina and now filed in Brazil. Would like to be kept updated on Soluplus® regulatory status or need further Information? Sign up here!
19. July 2018
Did you know that Soluplus® is already approved in generics in Taiwan and Argentina and now filed in Brazil? Check the submentioned Soluplus® one pager with updated regulatory status per country. Soluplus® Key Customer Benefits Outstanding solubilization properties, especially for poorly soluble APIs Enables bioavailability enhancement Ideal for hot melt extrusion and all standard granulation techniques Market proven solution for unique formulation challenges Would like to be kept updated on...
07. June 2018
Poly(2-ethyl-2-oxazoline) (PEOX), a biocompatible polymer considered as pseudo polypeptide, was introduced as a potential alternative to the commonly used polymer, poly(vinylpyrrolidone) (PVP) for the preparation of solid dispersion with a poorly soluble drug. Glipizide (GPZ), a BCS class II model drug, was selected for solubility and dissolution rate study. GPZ-polymer solid dispersions and physical mixtures were characterized and investigated by X-Ray diffractometry, differential scanning...