- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
05. May 2018
Along with the development of novel drug delivery systems the material science is also advancing. Conventional and novel synthetic or natural excipients provide opportunities to design dosage forms of the required features including their bioavailability.
12. April 2018
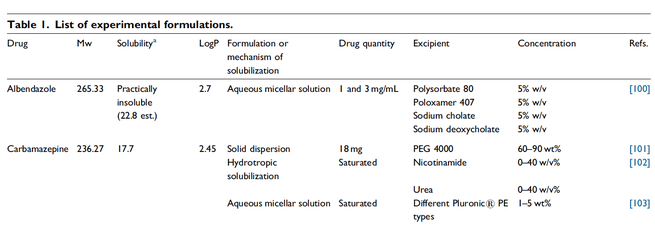
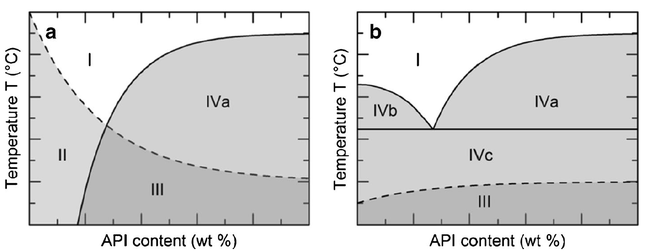
The oral bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs) can be improved by the preparation of amorphous solid dispersions (ASDs) where the API is dissolved in polymeric excipients. Desired properties of such ASDs like storage stability, dissolution behavior, and processability can be optimized by additional excipients. In this work, the influence of so-called low-molecular-weight excipients (LMWEs) on the phase behavior of ASDs was investigated.
26. January 2018
The understanding of amorphous solid dispersions has grown significantly in the past decade. This is evident from the number of approved commercial amorphous solid dispersion products. While amorphous formulation is considered an enabling technology, it has become the norm for formulating poorly soluble compounds.
08. November 2017
To investigate the effects of common nanosuspension-stabilizing excipients on the nature and temporal evolution of histopathological changes at intramuscular (i.m.) administration sites, 5 groups of 39 male rats per group received a single injection of 1 of the 5 analogous crystalline drug nanosuspensions containing 200 mg/ml of an antiviral compound with particle sizes of ±200 nm and identical vehicle compositions, except for the type of nanosuspension stabilizer.
15. September 2015
Hot-melt extrusion technology has been widely reported for producing amorphous solid dispersions of poorly water-soluble compounds. A number of studies revealed that enteric polymers containing ionizable groups are able to improve the physical stability and maintain drug supersaturation, thereby enhancing oral bioavailability. More
08. June 2015
The STEP stands for Safety and Toxicity of Excipients for Paediatrics. The Safety and Toxicity of Excipients for Paediatrics (STEP) Database is a user-designed resource developed by European Paediatric Formulation Initiative (EuPFI) and United States Paediatric Formulation Initiative (USPFI) in collaboration for storage and rapid/effortless access to the safety and toxicological data of excipients that is scattered over various sources and presents it in one freely accessible source. It is a...
03. June 2015
Press Release | ISOCHEM S.A.S. JUNE 03, 2015 Vert-Le-Petit, France: A senior scientist for specialist fine chemicals and API manufacturer ISOCHEM has called attention to the natural vitamin E derivative Tocophersolan (TPGS) as an innovative excipient in drug delivery.Dr. Yves Robin, ISOCHEM Vice President for R&D, reports that TPGS has surfactant properties that mean it can dramatically increase the concentration of an active substance in an aqueous matrix. Dr. Robin’s findings are...