- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
27. September 2017
Because of the multiple roles they play in drug product formulations, excipient quality is as important as that of the API, so standards are needed to ensure their purity and reliability.
15. September 2017
Excipients are critical for a drug to be effective. However, they can also cause great harm if their quality if poor. By creating standards for excipients, we play a key role in ensuring the purity of the whole drug. This column discusses the updates to and harmonization of excipient USP-NF standards and the importance of collaborative efforts with FDA and USP stakeholders. http://bit.ly/2wcT7Em
24. August 2017
Because excipients impact the effectiveness of drugs, reference standards are needed to ensure their quality. USP is working with partners to ensure excipient quality and protect and improve the public’s health
04. April 2017
08. February 2017
FDA and USP Workshop on Standards for Pharmaceutical Products February 27–28, 2017 USP Meetings Center, Rockville, MD USA More Information and Registration
30. August 2016
Abstract Pharmacopoeias, as standard references for pharmaceutical drug specifi cations and reference standards in the form of monographs, play a pivotal role to assure drug quality and safety. With emphasis on the activities concerning non-biological complex drugs (NBCDs), the mechanisms by which new monographs are introduced into the European and the US pharmacopoeias are presented. Introduction Over the last decades we came to realize that high molecular weight drugs of biological origin,...
21. June 2016
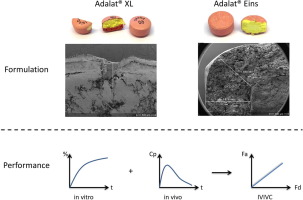
Aims Food intake is known to have various effects on gastrointestinal luminal conditions in terms of transit times, hydrodynamic forces and/or luminal fluid composition and can therefore affect the dissolution behavior of solid oral dosage forms. The aim of this study was to investigate and detect the dosage form-dependent food effect that has been observed for two extended-release formulations of nifedipine using in vitro dissolution tests. Methods Two monolithic extended release formulations,...
08. May 2016
As the second part of this series, this article focuses on the harmonization activities related to excipients under the work plan of the Pharmacopeial Discussion Group (PDG) that are also part of the USP Up-to-Date Initiative. As described in the first article, among the 19 FDA priority excipient monographs requested by FDA to be updated, fifteen (15) of them are in the PDG work plan among which eight (8) of these will be discussed in this article.1 Three expert panels were formed under the...
28. September 2015
The correction to the harmonized standard for Magnesium Stearate has been approved by the Pharmacopeial Discussion Group (PDG) as described in its PDG sign-off cover sheet. Having reached Stage 6 of the PDG process, the Magnesium Stearate monograph has been formally approved by the Monographs—Excipients Expert Committee in accordance with the Rules and Procedures of the Council of Experts. More