- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. August 2018
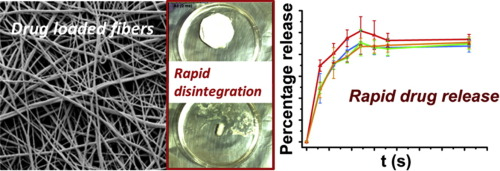
Increasing numbers of elderly people require multi-drug therapies. One route to improve adherence rates is to prepare fixed dose combinations (FDCs), in which multiple active ingredients are loaded into a single formulation. Here, we report the use of electrospinning to prepare fast-dissolving oral FDCs containing amlodipine besylate and valsartan, two drugs prescribed as FDCs for the treatment of hypertension. Electrospun fibers were prepared loaded with one or both drugs, using...
26. May 2018
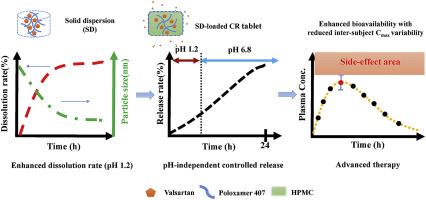
The aims of this work were to design pH-independent controlled release (CR) tablet containing nanonizing solid dispersion (SD) adsorbed on hydrophilic silica (Aeroperl® 300/30). Valsartan (VAL) was chosen to simultaneously modulate solubility and release rate due to its poor water solubility in low pH condition and short elimination half-life. Based on extensive equilibrium solubility and compatibility studies, poloxamer 407 was selected as a SD carrier. The melted mixtures of drug and...
11. December 2017
Use of a self-microemulsifying drug delivery system (SMEDDS) is widely known as one of the most effective approaches to overcome problems associated with low solubility and poor oral absorption of water-insoluble drugs. Previously, we have demonstrated that a super saturable SMEDDS (SuSMEDDS) greatly contributed to enhanced dissolution and oral absorption of valsartan (VST), a drug with poor solubility in water ...
21. October 2017
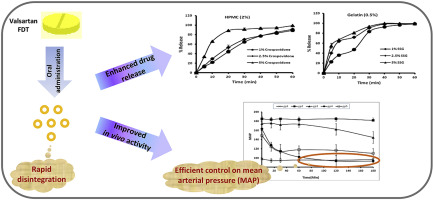
Fast disintegrating tablets (FDTs) dissolve or disintegrate in the mouth without the need of additional water. The aim of the present study was to formulate FDTs of Valsartan for the treatment of hypertension in children who could find difficulties in swallowing conventional solid dosage forms. The tablets were prepared by wet granulation technique. Superdisintegrants such as sodium starch glycolate (SSG) and crospovidone were optimized as 5% on the basis of least disintegration time. Different...
04. July 2016
Aim: To evaluate the use of mesoporous silica SYLOID® 244 FP to increase the dissolution rate of valsartan, antihypertensive poorly water soluble, Biopharmaceutical Classification System ClassII drug. Materials and Methods: Valsartan was adsorbed on and/or into SYLOID® 244 FP in the ratio of 1:0.5, 1:1, and 1:1.5 via a wetness impregnation method and then processed into tablet by direct compression. To investigate the interaction in between valsartan and SYLOID® 244 FP, X-ray powder...
13. April 2016
Co-amorphization has recently been shown to be a promising approach for stabilizing amorphous drugs and improving the dissolution rate of poorly water-soluble drugs. In this study, three basic amino acids were chosen as small molecular weight excipients to interact with the drug to form co-amorphous combinations. The co-amorphous combinations of valsartan (VAL) with l-histidine, l-arginine, and l-lysine were prepared by vibrational ball milling. Solid-state characterization with X-ray powder...
07. June 2015
Solid dispersion (SD) technique is a promising strategy to improve the solubility and dissolution of BCS class II drugs. However, only few products are marketed till today based on SD technology due to poor flow properties and stability. More...