- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. September 2018
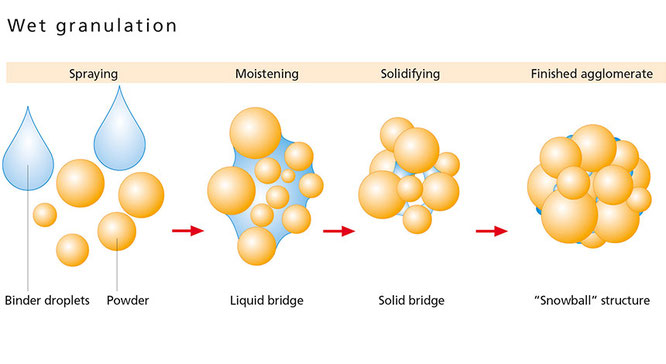
Wet granulation is a size-enlargement process applied in many industrial fields, such as pharmaceutical, nutraceutical, zootecnichal, to improve flowability and compressibility properties of powders. In this work analysis of the particle size distribution (PSD) of granules was performed to understand the phenomena involved during the granulation process and to optimize the operating conditions. Hydroxypropyl methylcellulose (HPMC) granules were produced spraying distilled water as liquid binder...
11. March 2018
Granulation is an integral step during pharmaceutical manufacturing of solid doses, which is usually followed by unit operations such as drying, milling, tableting and coating. Granulation is typically carried out to alleviate problems in powder handling, content non-uniformity, segregation and poor flow properties of powders. However, previous work carried out by Oka et. al. (Oka, Emady et al. 2015) points out that sometimes a wet granulation process might fail to give us a robust product ..
12. February 2018
Since decades, granulation is operational as a critical size enlargement process for powder agglomeration in tablet manufacturing. Dry granulation, melt granulation and wet granulation are some of the most common techniques utilized for granulation in the pharmaceutical industry.
19. January 2018
The authors studied six different grades of HPC to determine how they performed in high-shear and fluid-bed granulation.
17. October 2017
ARMOR PHARMA™ lactose monohydrate 350M is a fine milled powder of α-lactose monohydrate.
Higly compressible, this grade is mostly intended to be used in tablets formulation by Wet or Dry granulation.
18. August 2017
When tablet formulations are designed, it is necessary to understand “Tableting properties” and to determine the optimum type, grade, and amount of ingredients. “Tableting properties” consist of “Compressibility”, “Compactability”, and “Manufacturability”.
31. May 2017
Piracetam was investigated as a model API which is known to exhibit a number of different polymorphic forms. It is freely soluble in water so the possibility exists for polymorphic transformations to occur during wet granulation
16. May 2017
Abstract In this study, a Quality by Design (QbD) approach was used to identify the effect of formulation parameters in a twin screw wet extrusion granulation process for the manufacturing of ibuprofen (IBU) granules with increased dissolution rates. A fractional factorial Design of Experiment (DoE) was used to investigate the effect of the excipient composition, binder amount and liquid to solid (L/S) ratio (independent variables) on drug dissolution rates, median particle size diameter and...