- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
04. July 2018
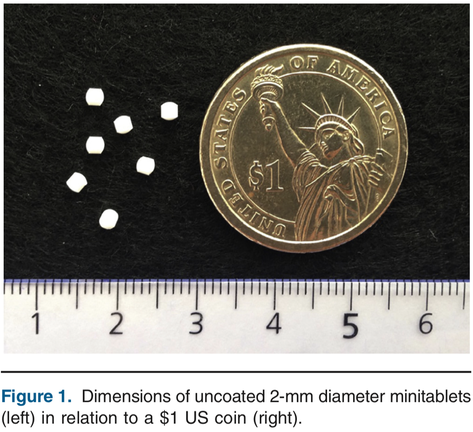
The objective of this study was to assess the acceptability and swallowability of several minitablets when administered as a unit dose compared with an equivalent dose of syrup in children aged 6 months to 5 years. Study design The acceptability and swallowability of multiple drug-free minitablets in comparison with glucose syrup was assessed in 372 children of 2 age groups (186 in age group 1 [6-23 months of age] and 186 in age group 2 [2-5 years of age]) in a randomized, 3-way, single...
30. August 2017
Abstract The acceptability of pediatric pharmaceutical products to patients and their caregivers can have a profound impact on the resulting therapeutic outcome. However, existing methodology and approaches used for acceptability assessments for pediatric products is fragmented, making robust and consistent product evaluations difficult. A pediatric formulation development workshop took place in Washington, DC in June 2016 through the University of Maryland’s Center of Excellence in...
17. July 2017
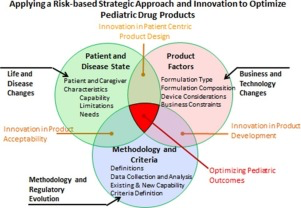
The selection and design of age-appropriate formulations intended for use in paediatric and geriatric patients are dependent on multiple factors affecting patient acceptability, safety and access.
24. March 2017
Abstract Introduction: Most conventional drug delivery systems are not acceptable for pediatric patients as they differ in their developmental status and dosing requirements from other subsets of the population. Technology platforms are required to aid the development of age-appropriate medicines to maximize patient acceptability while maintaining safety, efficacy, accessibility and affordability. Areas covered: The current approaches and novel developments in the field of age-appropriate drug...
20. March 2017
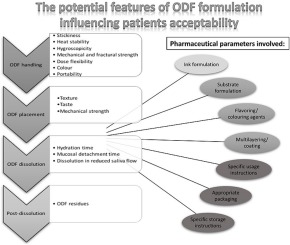
Abstract Orodispersible films (ODF) hold promise as a novel delivery method, with the potential to deliver tailored therapies to different patient populations. Together with the current trends and potential therapeutic areas such technology can address, some of the unmet needs in terms of quality aspects and manufacturing of conventional and novel (printed) ODF are discussed. Overall, this article aims to stimulate further research to fill the current knowledge gap between manufacturing and...
10. October 2016
Abstract A lack of evidence to guide the design of age-appropriate and acceptable dosage forms has been a longstanding knowledge gap in paediatric formulation development. The Children’s Acceptability of Oral Formulations (CALF) study captured end-user perceptions and practices with a focus on solid oral dosage forms, namely tablets, capsules, chewables, orodispersibles, multiparticulates (administered with food) and mini-tablets (administered directly into the mouth). A rigorous development...
20. August 2016
Objectives The aim of this review was to map the currently available evidence on acceptability of oral paediatric medicines to aid in the selection of suitable platform formulations for the development of new acceptable paediatric products. Methods This process used a defined search strategy of indexed publications and included methods to assess the quality of the evidence retrieved. Key findings Taste/palatability was the most extensively studied area of paediatric medicine acceptability yet...
11. August 2016
Many pediatric patients require medications that are not available in age appropriate formulations, especially those with cardiovascular diseases. The use of a suitable vehicle is critical for the preparation of extemporaneous formulations with the expected effect. Considering that palatability is essential to treatment adherence in children, we analyzed the acceptance of extemporaneous formulations of Captopril and Furosemide prepared using a developed vehicle in three flavors: neutral,...
10. August 2016
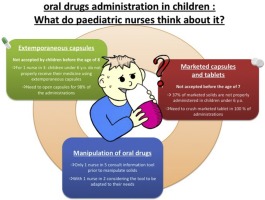
Abstract The purpose of this study was to interview paediatric nurses on administration issues using extemporaneous capsules and marketed capsules and tablets in children younger than 6 years old, based on most frequently administered drugs in six participating wards. The 59 responding nurses estimated respectively at 7.7 ± 1.7 and 7.3 ± 1.8 years the age from which children would properly swallow extemporaneous capsules and marketed solids, with 33% and 37% of nurses considering that...
28. July 2016
Abstract Multiparticulate formulations are composed of multiple solid dosage units which can be administered directly to the mouth or sprinkled on food. Oral grittiness (i.e. rough mouthfeel) may arise from the presence of particles in the mouth, limiting palatability. In this work, multiparticulate formulations were prepared by dispersion of spherical granules into orange flavoured vehicles thickened with hypromellose (HPMC) at different viscosities in order to assess oral perception of...