- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. August 2018
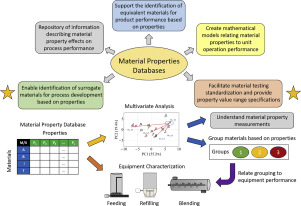
Material properties are known to have a significant impact on pharmaceutical manufacturing performance, particularly for solid product processes. Evaluating the performance of a specific material, for example an active pharmaceutical ingredient or excipient, is critical during development stages in order to determine the impact of material properties on the process. However, materials may be scarce during the early stages of process development due to high cost, unavailability, import...
17. August 2018
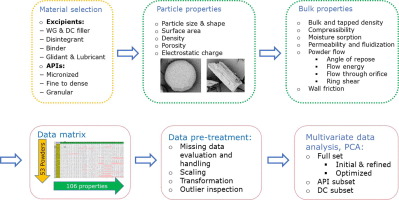
In current study a holistic material characterization approach was proposed and an extensive raw material property database was developed including a wide variety of APIs and excipients with different functionalities. In total 55 different materials were characterized and described by over 100 raw material descriptors related to particle size and shape distribution, specific surface area, bulk, tapped and true density, compressibility, electrostatic charge, moisture content, hygroscopicity,...
15. August 2018
Pharmaceutical tablets contain a variety of active substances and excipients which could be monitored by Raman spectroscopy/microscopy. Raman microscopic spectra are affected not only by the sample composition but also by preparation/measurement conditions. The character and variability of surface morphology (of tablet slices), the appropriate levels of focusing and other adjustable settings of the measurement instrumentation represent important parameters to be considered for obtaining...
08. July 2018
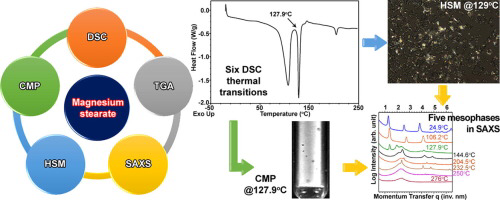
Magnesium stearate (MgSt) is the most commonly used excipient for oral solid dosage forms, yet there is significant commercial physicochemical variability that can lead to variable performance of critical product attributes. Differential scanning calorimetry (DSC) is often used as a quality control tool to characterize MgSt, but little data is available regarding the physicochemical relevance for the DSC thermograms. The main aim of this study was to decipher MgSt’s complex thermotropic...
07. July 2018
“If formulation science is the Cinderella of drug development, then excipients are the Cinderellas of formulation science”. R. C. Moreton, Finn Brit Consulting This chapter assumes some familiarity on the part of the reader with formulation and excipients, but familiarity can breed contempt. Application of Quality by Design requires thinking beyond pharmacopoeial compliance and fixed formulae, to avoid the inevitable quality problems arising from the use of complex ingredients in complex...
07. June 2018
Wet granulation is mostly used process for manufacturing matrix tablets. Compared to the direct compression method, it allows for a better flow and compressibility properties of compression mixtures. Granulation, including process parameters and tableting, can influence critical quality attributes (CQAs) of hydrophilic matrix tablets. One of the most important CQAs is the drug release profile. We studied the influence of granulation process parameters (type of nozzle and water quantity used as...
07. June 2018
3D printing evolved as a promising technique to improve individualization of drug therapy. In particular, when printing sustained release solid dosage forms, as for instance implants, inserts, and also tablets, estimation of the drug release profile in vivo is necessary. In most cases, corresponding analyses cannot be performed at hospital or community pharmacies. Therefore, the present study aimed to develop a sustained release drug delivery system produced via 3D printing, which allows dose...
07. May 2018
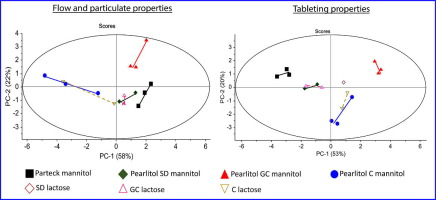
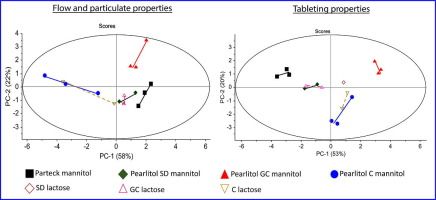
Appropriate selection of excipient grade during tablet formulation development depends on thorough knowledge in their compaction and flow properties. Each chemically unique pharmaceutical excipient is usually available in several commercial grades that are widely different in powder properties, which influence their performance for a specific formulation application. In this work, 11 grades of mannitol were systematically characterized, in terms of their particulate, flow and tableting...
02. May 2018
Appropriate selection of excipient grade during tablet formulation development depends on thorough knowledge in their compaction and flow properties. Each chemically unique pharmaceutical excipientis usually available in several commercial grades that are widely different in powder properties, which influence their performance for a specific formulation application. In this work, 11 grades of mannitol were systematically characterized, in terms of their particulate, flow and tableting...
29. November 2017
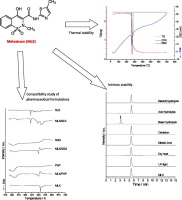
Meloxicam (MLX) is a non-steroidal anti-inflammatory cyclooxygenase (COX) inhibitor that is used to relieve inflammation and pain. MLX has a preferential affinity for COX-2, which is associated with a lower incidence of gastrointestinal side effects. The drug belongs to Class II of the Biopharmaceutical Classification System (BCS) in which dissolution is the limiting step of its bioavailability. In view of this classification, carrying out further studies regarding the compatibility of MLX with...