- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
01. October 2018
The occurrence of protein aggregation during bioprocessing steps such as purification, formulation and fill-finish, impacts yield and production costs, and must be controlled throughout themanufacturing process. Understanding aggregation mechanisms and developing mitigating strategies are imperative to ensure the clinical efficacy of the protein drug product and to reduce costs. Thiscommentary reflects on recent progress made in the field of monoclonal antibody (mAb) aggregation with...
25. July 2018
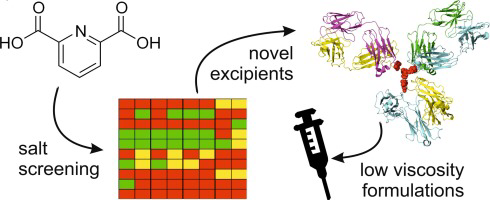
Concentrated monoclonal antibody (mAb) solutions can lead to high viscosity as a result of protein-protein interactions and pose challenges for manufacture. Dipicolinic acid (DPA, pyridine-2,6-dicarboxylic acid) is a potential excipient for reduction of protein solution viscosity and here we describe new DPA salts with improved aqueous solubility. Crystallinity and solubility screens identified ethanolamine and diethanolamine as two promising counterions which generated crystalline, high...
11. July 2018
New surfactant replacement offers a promising alternative to polysorbates for stabilizing proteins CAMBRIDGE, Mass. – July 11, 2018 – ReForm Biologics, a pharmaceutical technology company developing innovative biologic formulations to improve drug delivery and manufacturing, expanded its patent portfolio with the issuance of U.S. Patent No. 10,016,513 (the ‘513 patent). The patent, entitled “Stabilizing Excipients for Therapeutic Protein Formulations,” provides ReForm exclusive rights...
25. April 2018
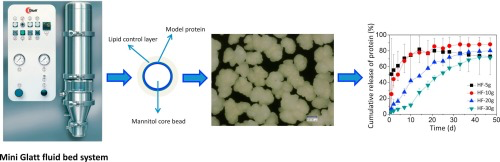
Parenteral sustained release systems for proteins which provide therapeutic levels over a longer period avoiding frequent administration, which preserve protein stability during manufacturing, storage and application and which are biodegradable and highly biocompatible in the body are intensively sought after. The aim of this study was to generate and study mannitol core microparticles loaded with a monoclonal antibody IgG1 and coated with lipid either hard fat or glyceryl stearate at different...
15. February 2017
Abstract Surfactants are widely used as stabilizers in the biopharmaceutical formulations to minimize protein aggregation. Under a fixed stress condition, the protecting and destabilizing effects of surfactants are hypothesized to be highly dependent on the species and concentrations of surfactants and mAb. Therefore, we here studied the aggregation-prevention and structure-perturbation effects of eight commonly used surfactants (Tw20, Tw80, Brij35, Chaps, TrX-100, SDS, Pluronic F68 and F127)...
04. July 2016
Abstract The technique of attaching the polymer polyethylene glycol (PEG), or PEGylation, has brought more than ten protein drugs into market. The surface conjugation of PEG on proteins prolongs their blood circulation time and reduces immunogenicity by increasing their hydrodynamic size and masking surface epitopes. Despite this success, an emerging body of literature highlights the presence of antibodies produced by the immune system that specifically recognize and bind to PEG (anti-PEG Abs),...
17. February 2016
The effects of an equimolar mixture of l-arginine and l-glutamate (Arg·Glu) on cell viability and cellular stress using in vitro cell culture systems are examined with reference to NaCl, in the context of monoclonal antibody formulation. Cells relevant to subcutaneous administration were selected: the human monocyte cell line THP-1, grown as a single cell suspension, and adherent human primary fibroblasts. For THP-1 cells, the mechanism of cell death caused by relatively high salt...