- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. May 2018
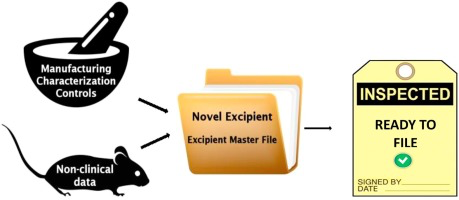
Novel excipients are indispensable in development of modern, advanced drug delivery systems and biotechnology-derived drugs. Although numerous novel excipients are developed for pharmaceutical use, they are not frequently seen in medicinal products due to the strict regulatory requirements and perception that their use makes new product evaluation more complex with risk of delays in the approval process. Regulators regard novel excipients as new substances and whenever new excipient is used in...
27. April 2018
The US Food and Drug Administration (FDA) approved over 700 generic drugs in 2015 alone, which was the highest to date, based on a report published by the FDA’s Office of Generic Drugs (OGD). The report also stated that the Agency approved 99 generic drugs in December 2015, which reflected FDA’s accomplishment report to Congress from March 2016.
04. January 2018
The year gone by saw the highest number of generic drug approvals by the US Food and Drug Administration (FDA), along with the most ever novel drugs (46); the most ever novel devices, and the first ever gene therapies. In 2017, 46 new molecular entities (NMEs) were approved by the FDA , and that excludes the pathbreaking CAR-T and gene therapies.
05. February 2015
The current practices for new excipient approval by regulatory agencies are grossly inefficient and hinder innovation. As many drugs’ patents expire, the excipient market is growing, and yet excipients lack independent approval processes separate from the drug. Natalie Healey asks Dr Chris Moreton from IPEC Americas what changes are necessary, and he discusses how investment in novel excipients may solve drug delivery challenges in future....