- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. April 2018
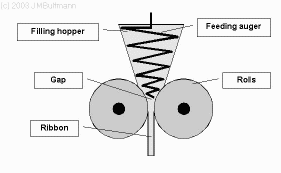
Metformin has a poor tabletability and flowability. Therefore, metformin is typically wet granulated with a binder before tableting. To save production costs, it would be desirable to implement a roll compaction/dry granulation (RCDG) process for metformin instead of using wet granulation. In order to implement RCDG, the efficiency of dry binders is crucial to ensure a high drug load and suitable properties of dry granules and tablets. This study evaluates dry granules manufactured by RCDG and...
30. April 2018
The overall objective of this work is to understand how excipient characteristics influence the drug product quality attributes and process performance of a continuous twin screw wet granulation process. The knowledge gained in this study is intended to be used for Quality by Design (QbD)-based formulation design and formulation optimization. Three principal components which represent the overarching properties of 8 selected pharmaceutical fillers were used as factors, whereas factors 4 and 5...
27. April 2018
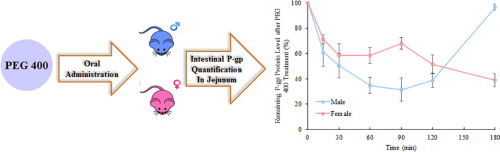
There is a growing body of evidence which suggests that formerly regarded “inert” pharmaceutical excipients have the potential to influence oral drug bioavailability. The solubilizing agent polyethylene glycol 400 (PEG 400), for instance, has a sex-specific effect on P-glycoprotein (P-gp)-mediated drug bioavailability. We hypothesized that such an effect could be via PEG-induced alteration of P-gp activity and/or expression to different extents in males and females. To test this hypothesis...
27. April 2018
The US Food and Drug Administration (FDA) approved over 700 generic drugs in 2015 alone, which was the highest to date, based on a report published by the FDA’s Office of Generic Drugs (OGD). The report also stated that the Agency approved 99 generic drugs in December 2015, which reflected FDA’s accomplishment report to Congress from March 2016.
27. April 2018
Although bioavailability of peptides administered through nasal route is still under 1% because of low membrane permeability, a short local residence time and a high metabolic turnover in the nasal epithelium but the richly supplied vascular nature of the nasal mucosa coupled with its high drug permeation makes the nasal route of administration attractive for many drugs including proteins and peptides. Thiolated polymers (thiomers) which are also recognized as mucoadhesive polymers, discovered...
27. April 2018
This article reviews regulatory considerations for companies wishing to develop drugs for delivery via the respiratory tract (e.g., by oral inhalation or intranasally) using molecules previously approved for a different therapeutic indication and/or a different delivery route. Conceptually, such repurposing has many medical and business advantages, but turning promising ideas into real products requires overcoming a number of practical challenges. Obtaining regulatory approval to market a...
25. April 2018
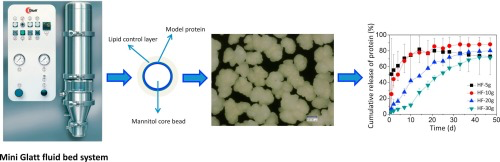
Parenteral sustained release systems for proteins which provide therapeutic levels over a longer period avoiding frequent administration, which preserve protein stability during manufacturing, storage and application and which are biodegradable and highly biocompatible in the body are intensively sought after. The aim of this study was to generate and study mannitol core microparticles loaded with a monoclonal antibody IgG1 and coated with lipid either hard fat or glyceryl stearate at different...
25. April 2018
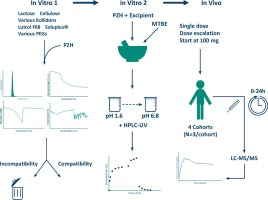
The anti-cancer drug pazopanib hydrochloride (PZH) has a very low aqueous solubility and a variable oral bioavailability. A new pharmaceutical formulation with an improved solubility may enhance the bioavailability and reduce the variability. A broad selection of polymer excipients was tested for their compatibility and solubilizing properties by conventional microscopic, thermal and spectrometric techniques. A wet milling and mixing technique was used to produce homogenous powder mixtures. The...
25. April 2018
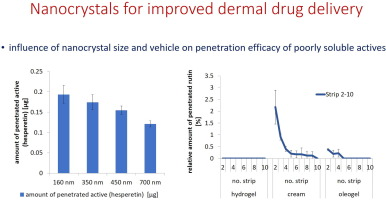
Nanocrystals are composed of 100% active and possess an increased aqueous solubility and dissolution velocity when compared to larger sized materials. Nanocrystals can be used to improve the bioavailability of poorly soluble actives not only for oral, but also for topical application. In this study nanocrystals of different sizes were produced and the influence of size on dermal penetration was investigated. The influence of different excipients and vehicles on the penetration efficacy upon...
25. April 2018
To support the practical implementation of the ICH Q3D guideline, which describes a risk-based approach to the control of elemental impurities in drug products, a consortium of pharmaceutical companies has established a database to collate the results of analytical studies of the levels of elemental impurities within pharmaceutical excipients. This database currently includes the results of 26723 elemental determinations for 201 excipients and represents the largest known, and still rapidly...