- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. July 2018
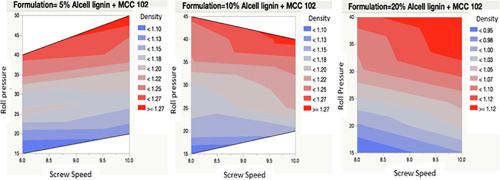
In this study, a process map was developed in an effort to improve the understanding of dry granulation of pharmaceutical excipients by roll compaction process, and to implement the quality-by-design (QbD) approach. Through development of the process map, a correlation was made between the critical process parameters (roll pressure, screw speed), and critical quality attributes (density of ribbons and granule size). This method reduces development time, quantity of materials required and cost....
28. November 2017
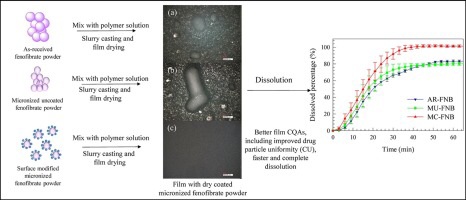
Recent work has established polymer strip films as a robust platform for delivery of poorly water-soluble drugs via slurry casting, in particular using stable drug nanosuspensions. Here, a simpler, robust method to directly incorporate dry micronized poorly water-soluble drug, fenofibrate (FNB), is introduced. As a major novelty, simultaneous surface modification using hydrophilic silica along with micronization was done using fluid energy mill (FEM) in order to reduce FNB hydrophobicity and...
22. April 2017
Abstract Mini-tablets have potential applications as a flexible drug delivery tool in addition to their generally perceived use as multi-particulates. That is, mini-tablets could provide flexibility in dose finding studies and/or allow for combination therapies in the clinic. Moreover, mini-tablets with well controlled quality attributes could be a prudent choice for administering solid dosage forms as a single unit or composite of multiple mini-tablets in patient populations with swallowing...
11. January 2017
Abstract With the implementation of quality by design (QbD), critical attributes of raw material (drug substance and excipients) are of significantly importance in pharmaceutical manufacturing process. It is desirable for the quality control of critical material attributes (CMAs) of excipients to ensure the quality of end product. This paper explored the feasibility of an at-line method for the quantitative analysis of hydroxypropoxy group in hydroxypropyl methylcellulose (HPMC) with near...
04. August 2016
Objectives: To develop orodispersible films (ODF) based on hydrophobic polymers with higher stability to ordinary environmental humidity conditions without compromising their fast disintegration time. Methods: A quality by design approach was applied to screen three different formulations each one based on a different hydrophobic polymer: polyvinyl acetate, methacrylate-based copolymer and shellac. The screening formulations were characterized regarding their mechanical properties, residual...
04. July 2016
Context: Orodispersible films have gained increasing relevance as a novel dosage form. Associated to their particular processing and multicomponent composition, there are a vast number of reasons to establish helpful development guidance, gathering simultaneously the recent pharmaceutical regulatory trends. Objective: This study aimed characterize marketed orodispersible films in order to provide essential information about a clear definition of product critical quality attributes (CQAs)....
05. August 2015
24. June 2015
Formulating drugs in the amorphous form is an attractive and promising strategy to overcome the poorly water soluble challenge commonly encountered in the newly discovered drug. The active research in the investigation of the properties and performance of drugs in the amorphous form has revealed the major challenges in developing amorphous formulations into marketable products. The three main challenges are development of analytical techniques to characterise the amorphous formulations,...
24. May 2015
Abstract The quality by design (QbD) initiative is promoting a better understanding of excipient performance and the identification of critical material attributes (CMAs). Despite microcrystalline cellulose (MCC) being one of the most popular direct compression binders, only a few studies attempted identifying its CMAs. These studies were based either on a limited number of samples or on MCC produced on a small scale and/or in conditions that deviate from those normally encountered in...